A multispecificity syntaxin homologue, Vam3p, essential for autophagic and biosynthetic protein transport to the vacuole
- PMID: 9245783
- PMCID: PMC2141632
- DOI: 10.1083/jcb.138.3.517
A multispecificity syntaxin homologue, Vam3p, essential for autophagic and biosynthetic protein transport to the vacuole
Abstract
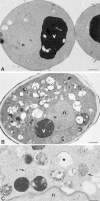
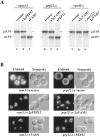
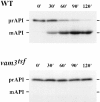
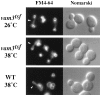
Protein transport in eukaryotic cells requires the selective docking and fusion of transport intermediates with the appropriate target membrane. t-SNARE molecules that are associated with distinct intracellular compartments may serve as receptors for transport vesicle docking and membrane fusion through interactions with specific v-SNARE molecules on vesicle membranes, providing the inherent specificity of these reactions. VAM3 encodes a 283-amino acid protein that shares homology with the syntaxin family of t-SNARE molecules. Polyclonal antiserum raised against Vam3p recognized a 35-kD protein that was associated with vacuolar membranes by subcellular fractionation. Null mutants of vam3 exhibited defects in the maturation of multiple vacuolar proteins and contained numerous aberrant membrane-enclosed compartments. To study the primary function of Vam3p, a temperature-sensitive allele of vam3 was generated (vam3(tsf)). Upon shifting the vam3(tsf) mutant cells to nonpermissive temperature, an immediate block in protein transport through two distinct biosynthetic routes to the vacuole was observed: transport via both the carboxypeptidase Y pathway and the alkaline phosphatase pathway was inhibited. In addition, vam3(tsf) cells also exhibited defects in autophagy. Both the delivery of aminopeptidase I and the docking/ fusion of autophagosomes with the vacuole were defective at high temperature. Upon temperature shift, vam3(tsf) cells accumulated novel membrane compartments, including multivesicular bodies, which may represent blocked transport intermediates. Genetic interactions between VAM3 and a SEC1 family member, VPS33, suggest the two proteins may act together to direct the docking and/or fusion of multiple transport intermediates with the vacuole. Thus, Vam3p appears to function as a multispecificity receptor in heterotypic membrane docking and fusion reactions with the vacuole. Surprisingly, we also found that overexpression of the endosomal t-SNARE, Pep12p, suppressed vam3Delta mutant phenotypes and, likewise, overexpression of Vam3p suppressed the pep12Delta mutant phenotypes. This result indicated that SNAREs alone do not define the specificity of vesicle docking reactions.
Figures





References
-
- Banfield DK, Lewis MJ, Pelham HR. A SNARE-like protein required for traffic through the Golgi complex. Nature (Lond) 1995;375:806–809. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

