Spindle assembly in Xenopus egg extracts: respective roles of centrosomes and microtubule self-organization
- PMID: 9245790
- PMCID: PMC2141625
- DOI: 10.1083/jcb.138.3.615
Spindle assembly in Xenopus egg extracts: respective roles of centrosomes and microtubule self-organization
Abstract
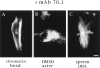
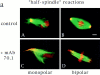
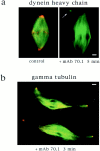
In Xenopus egg extracts, spindles assembled around sperm nuclei contain a centrosome at each pole, while those assembled around chromatin beads do not. Poles can also form in the absence of chromatin, after addition of a microtubule stabilizing agent to extracts. Using this system, we have asked (a) how are spindle poles formed, and (b) how does the nucleation and organization of microtubules by centrosomes influence spindle assembly? We have found that poles are morphologically similar regardless of their origin. In all cases, microtubule organization into poles requires minus end-directed translocation of microtubules by cytoplasmic dynein, which tethers centrosomes to spindle poles. However, in the absence of pole formation, microtubules are still sorted into an antiparallel array around mitotic chromatin. Therefore, other activities in addition to dynein must contribute to the polarized orientation of microtubules in spindles. When centrosomes are present, they provide dominant sites for pole formation. Thus, in Xenopus egg extracts, centrosomes are not necessarily required for spindle assembly but can regulate the organization of microtubules into a bipolar array.
Figures













References
-
- Albertson DG, Thomson JN. Segregation of holocentric chromosomes at meiosis in the nematode, Caenorhabditis elegans. . Chromosome Res. 1993;1:15–26. - PubMed
-
- Bajer AS, Mole BJ. Asters, poles, and transport properties within spindlelike microtubule arrays. Cold Spring Harbor Symp Quant Biol. 1982;1:263–283. - PubMed
-
- Bastmeyer M, Steffen W, Fuge H. Immunostaining of spindle components in tipulid spermatocytes using a serum against pericentriolar material. Eur J Cell Biol. 1986;42:305–310. - PubMed
-
- Belmont LD, Hyman AA, Sawin KE, Mitchison TJ. Real-time visualization of cell cycle-dependent changes in microtubule dynamics in cytoplasmic extracts. Cell. 1990;62:579–589. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

