Introduction of a glycosylation site into a secreted protein provides evidence for an alternative antigen processing pathway: transport of precursors of major histocompatibility complex class I-restricted peptides from the endoplasmic reticulum to the cytosol
- PMID: 9254646
- PMCID: PMC2199039
- DOI: 10.1084/jem.186.4.479
Introduction of a glycosylation site into a secreted protein provides evidence for an alternative antigen processing pathway: transport of precursors of major histocompatibility complex class I-restricted peptides from the endoplasmic reticulum to the cytosol
Abstract
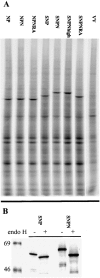
We found that the presentation of a H-2Kd-restricted determinant from influenza virus nucleoprotein (NP) to T cells is strictly dependent on expression of the transporter associated with antigen presentation (TAP), regardless of whether NP is expressed as a cytosolic or secreted NP (SNP). Introducing an N-linked glycosylation site into the determinant selectively reduced presentation of SNP. This indicates that glycosylation does not interfere with TAP-transported peptides, and therefore that cytosolic peptides derived from SNP must have been exposed to the glycosylation machinery of the endoplasmic reticulum (ER) before their existence in the cytosol. Based on these findings, we propose that TAP-dependent processing of at least some ER-targeted proteins entails the reimportation of protein from the secretory pathway to the cytosol, where the protein is processed via the classical pathway.
Figures




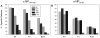
References
-
- York I, Rock KL. Antigen processing and presentation by the class I major histocompatibility complex. Annu Rev Immunol. 1996;14:369–396. - PubMed
-
- Heemels M-T, Ploegh H. Generation, translocation, and presentation of MHC class-I restricted peptides. Annu Rev Biochem. 1995;64:463–491. - PubMed
-
- Suh W-K, Cohen-Doyle MF, Fruh K, Wang K, Peterson PA, Williams DB. Interaction of MHC class I molecules with the transporter associated with antigen processing. Science (Wash DC) 1994;264:1322–1326. - PubMed
-
- Ortmann B, Androlewicz MJ, Cresswell P. MHC class I/β2-microglobulin complexes associate with TAP transporters before peptide binding. Nature (Lond) 1994;368:864–867. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

