Src homology 2 protein tyrosine phosphatase (SHPTP2)/Src homology 2 phosphatase 2 (SHP2) tyrosine phosphatase is a positive regulator of the interleukin 5 receptor signal transduction pathways leading to the prolongation of eosinophil survival
- PMID: 9254654
- PMCID: PMC2199030
- DOI: 10.1084/jem.186.4.561
Src homology 2 protein tyrosine phosphatase (SHPTP2)/Src homology 2 phosphatase 2 (SHP2) tyrosine phosphatase is a positive regulator of the interleukin 5 receptor signal transduction pathways leading to the prolongation of eosinophil survival
Abstract
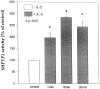
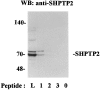
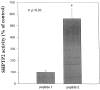
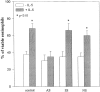
Interleukin-5 (IL-5) regulates the growth and function of eosinophils. It induces rapid tyrosine phosphorylation of Lyn and Jak2 tyrosine kinases. The role of tyrosine phosphatases in IL-5 signal transduction has not been investigated. In this study, we provide first evidence that SH2 protein tyrosine phosphatase 2 (SHPTP2) phosphotyrosine phosphatase plays a key role in prevention of eosinophil death by IL-5. We found that IL-5 produced a rapid activation and tyrosine phosphorylation of SHPTP2 within 1 min. The tyrosine phosphorylated SHPTP2 was complexed with the adapter protein Grb2 in IL-5-stimulated eosinophils. Furthermore, SHPTP2 appeared to physically associate with beta common (betac) chain of the IL-5 receptor (IL-5betacR). The association of SHPTP2 with IL-5betacR was reconstituted using a synthetic phosphotyrosine-containing peptide, betac 605-624, encompassing tyrosine (Y)612. The binding to the phosphotyrosine-containing peptide increased the phosphatase activity of SHPTP2, whereas the same peptide with the phosphorylated Y612--> F mutation did not activate SHPTP2. Only SHPTP2 antisense oligonucleotides, but not sense SHPTP2, could inhibit tyrosine phosphorylation of microtubule-associated protein kinase, and reverse the eosinophil survival advantage provided by IL-5. Therefore, we conclude that the physical association of SHPTP2 with the phosphorylated betac receptor and Grb2 and its early activation are required for the coupling of the receptor to the Ras signaling pathway and for prevention of eosinophil death by IL-5.
Figures

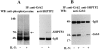
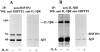


Similar articles
-
Interleukin (IL)-3 and granulocyte/macrophage colony-stimulating factor, but not IL-4, induce tyrosine phosphorylation, activation, and association of SHPTP2 with Grb2 and phosphatidylinositol 3'-kinase.J Biol Chem. 1994 Sep 23;269(38):23764-8. J Biol Chem. 1994. PMID: 7522233
-
Protein-tyrosine-phosphatase SHPTP2 is a required positive effector for insulin downstream signaling.Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):664-8. doi: 10.1073/pnas.92.3.664. Proc Natl Acad Sci U S A. 1995. PMID: 7531337 Free PMC article.
-
Involvement of Src-homology-2-domain-containing protein-tyrosine phosphatase 2 in T cell activation.Eur J Biochem. 1996 May 1;237(3):736-42. doi: 10.1111/j.1432-1033.1996.0736p.x. Eur J Biochem. 1996. PMID: 8647120
-
The insulin receptor substrate 1 associates with phosphotyrosine phosphatase SHPTP2 in liver and muscle of rats.Braz J Med Biol Res. 1998 Nov;31(11):1409-13. doi: 10.1590/s0100-879x1998001100007. Braz J Med Biol Res. 1998. PMID: 9921276 Review.
-
The mechanism of IL-5 signal transduction.Am J Physiol. 1998 Sep;275(3):C623-33. doi: 10.1152/ajpcell.1998.275.3.C623. Am J Physiol. 1998. PMID: 9730944 Review.
Cited by
-
Enzalutamide-Induced Feed-Forward Signaling Loop Promotes Therapy-Resistant Prostate Cancer Growth Providing an Exploitable Molecular Target for Jak2 Inhibitors.Mol Cancer Ther. 2020 Jan;19(1):231-246. doi: 10.1158/1535-7163.MCT-19-0508. Epub 2019 Sep 23. Mol Cancer Ther. 2020. PMID: 31548294 Free PMC article.
-
Lyn, Jak2, and Raf-1 kinases are critical for the antiapoptotic effect of interleukin 5, whereas only Raf-1 kinase is essential for eosinophil activation and degranulation.J Exp Med. 1998 Aug 3;188(3):421-9. doi: 10.1084/jem.188.3.421. J Exp Med. 1998. PMID: 9687520 Free PMC article.
-
SHP-2 in Lymphocytes' Cytokine and Inhibitory Receptor Signaling.Front Immunol. 2019 Oct 25;10:2468. doi: 10.3389/fimmu.2019.02468. eCollection 2019. Front Immunol. 2019. PMID: 31708921 Free PMC article. Review.
-
Regulation of interleukin-3-induced substrate phosphorylation and cell survival by SHP-2 (Src-homology protein tyrosine phosphatase 2).Biochem J. 2003 Nov 15;376(Pt 1):147-57. doi: 10.1042/BJ20031160. Biochem J. 2003. PMID: 12935294 Free PMC article.
-
Cross-talk between ICAM-1 and granulocyte-macrophage colony-stimulating factor receptor signaling modulates eosinophil survival and activation.J Immunol. 2008 Mar 15;180(6):4182-90. doi: 10.4049/jimmunol.180.6.4182. J Immunol. 2008. PMID: 18322230 Free PMC article.
References
-
- Corrigan CJ, Kay AB. T cells and eosinophils in the pathogenesis of asthma. Immunol Today. 1992;13:501–503. - PubMed
-
- Owen, W.F., Jr., and K.F. Austen. 1994. Cytokine regulation of eosinophil-mediated inflammatory reactions by modulation of eosinophil programmed cell death and subsequent priming for augmented function. In Eosinophils in Allergy and Inflammation. G.J. Gleich and A.B. Kay, editors. Marcel Decker, New York. 239–253.
-
- Tavernier J, Devos R, Cornelis S, Tuypens T, Van der Heyden J, Fiers W, Plaetnick G. A human high affinity interleukin-5 receptor (IL-5) is composed of an IL-5 specific α chain and a β chain shared with the receptor for GM-CSF. Cell. 1991;66:1175–1180. - PubMed
-
- Kanakura Y, Druker B, Connistra SA, Furukawa SA, Torinoko Y, Griffin JD. Signal transduction of the human granulocyte macrophage colony-stimulating factor and interleukin 3 receptors involves tyrosine phosphorylation of a common set of cytoplasmic proteins. Blood. 1990;76:706–711. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

