Vascular adhesion protein 1 (VAP-1) mediates lymphocyte subtype-specific, selectin-independent recognition of vascular endothelium in human lymph nodes
- PMID: 9254657
- PMCID: PMC2199032
- DOI: 10.1084/jem.186.4.589
Vascular adhesion protein 1 (VAP-1) mediates lymphocyte subtype-specific, selectin-independent recognition of vascular endothelium in human lymph nodes
Abstract
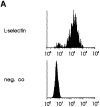
Interactions between lymphocyte surface receptors and their ligands on vascular endothelial cells regulate the exit of lymphocytes from the circulation. Distinct subsets of mononuclear cells bind to high endothelial venules (HEVs) in different lymphoid organs to a different extent, but the molecular mechanisms behind this selectivity have remained poorly characterized. Here we show that vascular adhesion protein-1 (VAP-1) mediates subtype-specific binding of CD8-positive T cells and natural killer cells to human endothelium. VAP-1-dependent, oligosaccharide-dependent peripheral lymph node (PLN) HEV adhesion under shear was independent of L-selectin, P-selectin glycoprotein ligand 1, and alpha4 integrins, the known lymphocyte receptors involved in the initial recognition of endothelial cells. PLN HEV adhesion was also critically dependent on peripheral lymph node vascular addressins (PNAds), but lymphocyte L-selectin was absolutely required for PNAd binding. Most lymphocytes relied on both PNAd and VAP-1 in HEV binding. The overlapping function of L-selectin ligands and VAP-1 in PLN introduces a new control point into the lymphocyte extravasation process. Finally, intravital microscopy revealed that VAP-1 is involved in initial interactions between human lymphocytes and endothelial cells in inflamed rabbit mesenterial venules in vivo. In conclusion, VAP-1 is a novel contact-initiating ligand that discriminates between different subpopulations of mononuclear cells and is an appealing target for selective modulation of adhesion of CD8- and CD16-positive effector cells.
Figures




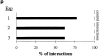
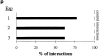
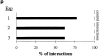
References
-
- Gowans JL, Knight EJ. The route of re-circulation of lymphocytes in rat. Proc R Soc Lond Ser B. 1964;159:257–282. - PubMed
-
- Stevens SK, Weissman IL, Butcher EC. Differences in the migration of B and T lymphocytes: organ-selective localization in vivoand the role of lymphocyte– endothelial cell recognition. J Immunol. 1982;128:844–851. - PubMed
-
- Fossum S, Smith ME, Ford WL. The recirculation of T and B lymphocytes in the athymic, nude rat. Scand J Immunol. 1983;17:551–557. - PubMed
-
- Kraal G, Weissman IL, Butcher EC. Differences in in vivo distribution and homing of T cell subsets to mucosal vs.non-mucosal lymphoid organs. J Immunol. 1983;130:1097–1102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

