Kinetics of eotaxin generation and its relationship to eosinophil accumulation in allergic airways disease: analysis in a guinea pig model in vivo
- PMID: 9254658
- PMCID: PMC2199038
- DOI: 10.1084/jem.186.4.601
Kinetics of eotaxin generation and its relationship to eosinophil accumulation in allergic airways disease: analysis in a guinea pig model in vivo
Abstract
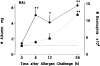
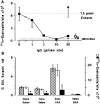
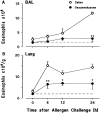
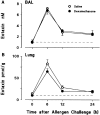
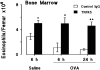
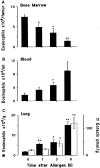
Challenge of the airways of sensitized guinea pigs with aerosolized ovalbumin resulted in an early phase of microvascular protein leakage and a delayed phase of eosinophil accumulation in the airway lumen, as measured using bronchoalveolar lavage (BAL). Immunoreactive eotaxin levels rose in airway tissue and BAL fluid to a peak at 6 h falling to low levels by 12 h. Eosinophil numbers in the tissue correlated with eotaxin levels until 6 h but eosinophils persisted until the last measurement time point at 24 h. In contrast, few eosinophils appeared in BAL over the first 12 h, major trafficking through the airway epithelium occurring at 12-24 h when eotaxin levels were low. Constitutive eotaxin was present in BAL fluid. Both constitutive and allergen-induced eosinophil chemoattractant activity in BAL fluid was neutralized by an antibody to eotaxin. Allergen-induced eotaxin appeared to be mainly in airway epithelium and macrophages, as detected by immunostaining. Allergen challenge of the lung resulted in a rapid release of bone marrow eosinophils into the blood. An antibody to IL-5 suppressed bone marrow eosinophil release and lung eosinophilia, without affecting lung eotaxin levels. Thus, IL-5 and eotaxin appear to cooperate in mediating a rapid transfer of eosinophils from the bone marrow to the lung in response to allergen challenge.
Figures



References
-
- Metzger WJ, Zavala D, Richerson HB, Moseley P, Iwamota P, Monick M, Sjoerdsma K, Hunninghake GW. Local allergen challenge and bronchoalveolar lavage of allergic asthmatic lungs. Am Rev Respir Dis. 1987;135:433–440. - PubMed
-
- de Monchy JGR, Kauffman HF, Venge P, Koeter GH, Jansen HM, Sluiter HJ, de Vries K. Bronchoalveolar eosinophilia during allergen-induced late asthmatic reactions. Am Rev Respir Dis. 1985;131:373–376. - PubMed
-
- Bousquet J, Chanez P, Lacoste JY, Barneon G, Ghavanian MN, Enander I, Venge P, Ahlstedt S, Simony-Lafontaine J, Godard P, Michel P-B. Eosinophilic inflammation in asthma. N Engl J Med. 1990;323:1033–1039. - PubMed
-
- Bradley BL, Azzawi M, Jacobson M, Assoufi B, Collins JV, Irani A-MA, Schwartz LB, Durham SR, Jeffery PK, Kay AB. Eosinophils, T-lymphocytes, mast cells, neutrophils, and macrophages in bronchial biopsy specimens from atopic subjects with asthma: comparison with biopsy specimens from atopic subjects without asthma and normal control subjects and relationship to bronchial hyperresponsiveness. J Allergy Clin Immunol. 1991;88:661–674. - PubMed
-
- Gleich GJ, Flavahan NA, Fujisawa T, Vanhoutte PM. The eosinophil as a mediator of damage to respiratory epithelium: a model for bronchial hyperreactivity. J Allergy Clin Immunol. 1988;81:776–781. - PubMed

