Targeted transduction of CNS neurons with adenoviral vectors carrying neurotrophic factor genes confers neuroprotection that exceeds the transduced population
- PMID: 9254662
- PMCID: PMC6573127
- DOI: 10.1523/JNEUROSCI.17-17-06504.1997
Targeted transduction of CNS neurons with adenoviral vectors carrying neurotrophic factor genes confers neuroprotection that exceeds the transduced population
Abstract
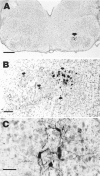
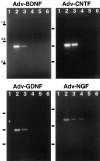
Application of neurotrophic factors (NFs) to the cut stump of motor nerves of neonatal rats confers neuroprotection from trauma-induced neuronal death. To test whether motoneurons are capable of responding to endogenously produced NFs, facial motoneurons were genetically modified in vivo to express several NFs and then tested for their response to peripheral nerve damage. Replication-defective adenoviral vectors [Adv. Rous sarcoma virus (RSV)-nf] representing three families of NFs were constructed that carried genes for brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF), glial cell-derived neurotrophic factor (GDNF), and nerve growth factor. Media from cultured cells transduced with Adv. RSV-nf contained NFs that supported the survival of cultured chick sensory neurons in the same manner as recombinant NF standards. When Adv.RSV-nf or an adenoviral vector containing the beta-galactosidase gene (Adv.RSV-beta-gal) were injected into the facial muscles of neonatal rats the vectors were retrogradely transported to the facial nucleus where the NFs or beta-gal were expressed. A fraction (approximately 10%) of the neurons were transduced as demonstrated by reverse transcriptase-PCR, histochemistry, and immunocytochemistry. In the case of Adv.RSV-BDNF, Adv.RSV-CNTF, and Adv.RSV-GDNF, a significant portion of the facial nucleus neurons was protected, 16.5, 18.2, and 53.3%, respectively, from death after axotomy, showing that neurons are capable of transporting the Adv. RSV-nf, expressing the recombinant NF genes, and responding to the NFs. In the case of Adv.RSV-GDNF, a greater number of facial nucleus motoneurons survived than were transduced, indicating that neighboring untransduced neurons were protected by the GDNF expressed by the transduced neurons by a paracrine mechanism.
Figures



References
-
- Akli S, Caillaud C, Vigne E, Stratford-Perricaudet LD, Poenaru L, Perricaudet M, Kahn A, Peschanski MR. Transfer of a foreign gene into the brain using adenovirus vectors. Nat Genet. 1993;3:224–228. - PubMed
-
- Baumgartner BJ, Barnes EM., Jr . Analysis of neuronal mRNA levels by quantitative reverse transcriptase-polymerase chain reaction. In: Wouterlood FG, editor. Neuroscience protocols, Module 4, Sec 20. Elsevier Science; Amsterdam: 1994. pp. 1–13.
-
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. - PubMed
-
- Davidson BL, Allen ED, Kozarsky KF, Wilson JM, Roessler BJ. A model system for in vivo gene transfer into the central nervous system using an adenoviral vector. Nat Genet. 1993;3:219–223. - PubMed
-
- Davies AM. Neurotrophic factor bioassay using dissociated neurons. In: Rush RA, editor. Nerve growth factors. Wiley; New York: 1989. pp. 95–109.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
