Slow recovery from inactivation of Na+ channels underlies the activity-dependent attenuation of dendritic action potentials in hippocampal CA1 pyramidal neurons
- PMID: 9254663
- PMCID: PMC6573147
- DOI: 10.1523/JNEUROSCI.17-17-06512.1997
Slow recovery from inactivation of Na+ channels underlies the activity-dependent attenuation of dendritic action potentials in hippocampal CA1 pyramidal neurons
Abstract
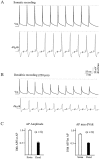
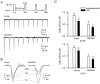
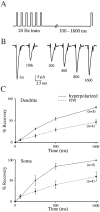
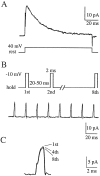
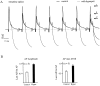
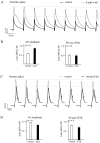
Na+ action potentials propagate into the dendrites of pyramidal neurons driving an influx of Ca2+ that seems to be important for associative synaptic plasticity. During repetitive (10-50 Hz) firing, dendritic action potentials display a marked and prolonged voltage-dependent decrease in amplitude. Such a decrease is not apparent in somatic action potentials. We investigated the mechanisms of the different activity dependence of somatic and dendritic action potentials in CA1 pyramidal neurons of adult rats using whole-cell and cell-attached patch-clamp methods. There were three main findings. First, dendritic Na+ currents decreased in amplitude when repeatedly activated by brief (2 msec) depolarizations. Recovery was slow and voltage-dependent. Second, Na+ currents decreased much less in somatic than in dendritic patches. Third, although K+ currents remained constant during trains, K+ currents were necessary for dendritic action potential amplitude to decrease in whole-cell experiments. These results suggest that regional differences in Na+ and K+ channels determine the differences in the activity dependence of somatic and dendritic action potential amplitudes.
Figures
References
-
- Callaway JC, Ross WN. Frequency dependent propagation of sodium action potentials in dendrites of hippocampal CA1 pyramidal neurons. J Neurophysiol. 1995;74:1395–1403. - PubMed
-
- Cantrell AR, Ma JY, Scheurer T, Catterall WA. Muscarinic modulation of sodium current by activation of protein kinase C in rat hippocampal neurons. Neuron. 1996;16:1019–1026. - PubMed
-
- Catterall WA. Cellular and molecular biology of voltage-gated sodium channels. Physiol Rev. 1992;72:S15–S48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
