Adenoviral vector-mediated expression of B-50/GAP-43 induces alterations in the membrane organization of olfactory axon terminals in vivo
- PMID: 9254670
- PMCID: PMC6573140
- DOI: 10.1523/JNEUROSCI.17-17-06575.1997
Adenoviral vector-mediated expression of B-50/GAP-43 induces alterations in the membrane organization of olfactory axon terminals in vivo
Abstract
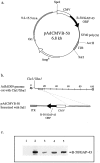
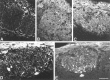
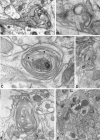
B-50/GAP-43 is an intraneuronal membrane-associated growth cone protein with an important role in axonal growth and regeneration. By using adenoviral vector-directed expression of B-50/GAP-43 we studied the morphogenic action of B-50/GAP-43 in mature primary olfactory neurons that have established functional synaptic connections. B-50/GAP-43 induced gradual alterations in the morphology of olfactory synapses. In the first days after overexpression, small protrusions originating from the preterminal axon shaft and from the actual synaptic bouton were formed. With time the progressive formation of multiple ultraterminal branches resulted in axonal labyrinths composed of tightly packed sheaths of neuronal membrane. Thus, B-50/GAP-43 is a protein that can promote neuronal membrane expansion at synaptic boutons. This function of B-50/GAP-43 suggests that this protein may subserve an important role in ongoing structural synaptic plasticity in adult neurons and in neuronal membrane repair after injury to synaptic fields.
Figures




References
-
- Aigner L, Arber S, Kapfhammer JP, Laux T, Schneider C, Botteri F, Brenner H-R, Caroni P. Overexpression of the neural growth associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell. 1995;83:269–278. - PubMed
-
- Akli S, Caillaud C, Vigne E, Stratford-Perricaudet LD, Poenaru L, Perricaudet M, Kahn A, Peschanski MR. Transfer of a foreign gene into the brain using adenovirus vectors. Nat Genet. 1993;3:224–228. - PubMed
-
- Aloyo VJ, Zwiers H, Gispen WH. Phosphorylation of B-50 protein by calcium-activated, phospholipid-dependent protein kinase and B-50 protein kinase. J Neurochem. 1983;41:649–653. - PubMed
-
- Bailey MS, Shipley MT. Astrocyte subtypes in the rat olfactory bulb; morphological heterogeneity and differential laminar distribution. J Comp Neurol. 1993;328:501–526. - PubMed
-
- Bajocchi G, Feldman SH, Crystal RG, Mastrangeli A. Direct in vivo gene transfer to ependymal cells in the central nervous system using recombinant adenovirus vectors. Nat Genet. 1993;3:229–334. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
