Assembly of GABAA receptors composed of alpha1 and beta2 subunits in both cultured neurons and fibroblasts
- PMID: 9254671
- PMCID: PMC6573131
- DOI: 10.1523/JNEUROSCI.17-17-06587.1997
Assembly of GABAA receptors composed of alpha1 and beta2 subunits in both cultured neurons and fibroblasts
Abstract
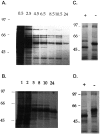
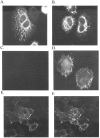
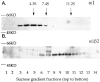
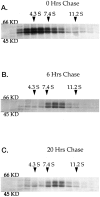
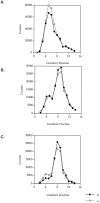
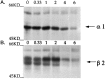
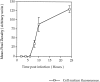
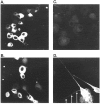
GABAA receptors are believed to be pentameric hetero-oligomers, which can be constructed from six subunits (alpha, beta, gamma, delta, epsilon, and rho) with multiple members, generating a large potential for receptor heterogeneity. The mechanisms used by neurons to control the assembly of these receptors, however, remain unresolved. Using Semliki Forest virus expression we have analyzed the assembly of 9E10 epitope-tagged receptors comprising alpha1 and beta2 subunits in baby hamster kidney cells and cultured superior cervical ganglia neurons. Homomeric subunits were retained within the endoplasmic reticulum, whereas heteromeric receptors were able to access the cell surface in both cell types. Sucrose density gradient fractionation demonstrated that the homomeric subunits were incapable of oligomerization, exhibiting 5 S sedimentation coefficients. Pulse-chase analysis revealed that homomers were degraded, with half-lives of approximately 2 hr for both the alpha1((9E10)) and beta2((9E10)) subunits. Oligomerization of the alpha1((9E10)) and beta2((9E10)) subunits was evident, as demonstrated by the formation of a stable 9 S complex, but this process seemed inefficient. Interestingly the appearance of cell surface receptors was slow, lagging up to 6 hr after the formation of the 9 S receptor complex. Using metabolic labeling a ratio of alpha1((9E10)):beta2((9E10)) of 1:1 was found in this 9 S fraction. Together the results suggest that GABAA receptor assembly occurs by similar mechanisms in both cell types, with retention in the endoplasmic reticulum featuring as a major control mechanism to prevent unassembled receptor subunits accessing the cell surface.
Figures


Similar articles
-
Endoplasmic reticulum retention and associated degradation of a GABAA receptor epilepsy mutation that inserts an aspartate in the M3 transmembrane segment of the alpha1 subunit.J Biol Chem. 2005 Nov 11;280(45):37995-8004. doi: 10.1074/jbc.M508305200. Epub 2005 Aug 25. J Biol Chem. 2005. PMID: 16123039
-
Identification of amino acid residues within GABA(A) receptor beta subunits that mediate both homomeric and heteromeric receptor expression.J Neurosci. 1999 Aug 1;19(15):6360-71. doi: 10.1523/JNEUROSCI.19-15-06360.1999. J Neurosci. 1999. PMID: 10414965 Free PMC article.
-
Identification of residues within GABA(A) receptor alpha subunits that mediate specific assembly with receptor beta subunits.J Neurosci. 2000 Feb 15;20(4):1297-306. doi: 10.1523/JNEUROSCI.20-04-01297.2000. J Neurosci. 2000. PMID: 10662819 Free PMC article.
-
Multiple assembly signals in gamma-aminobutyric acid (type A) receptor subunits combine to drive receptor construction and composition.Biochem Soc Trans. 2003 Aug;31(Pt 4):875-9. doi: 10.1042/bst0310875. Biochem Soc Trans. 2003. PMID: 12887325 Review.
-
The diversity of GABA(A) receptor subunit distribution in the normal and Huntington's disease human brain.Adv Pharmacol. 2015;73:223-64. doi: 10.1016/bs.apha.2014.11.010. Epub 2015 Jan 17. Adv Pharmacol. 2015. PMID: 25637443 Review.
Cited by
-
mGluR2/3 agonist LY379268 rescues NMDA and GABAA receptor level deficits induced in a two-hit mouse model of schizophrenia.Psychopharmacology (Berl). 2016 Apr;233(8):1349-59. doi: 10.1007/s00213-016-4230-0. Epub 2016 Feb 10. Psychopharmacology (Berl). 2016. PMID: 26861891
-
Regulation of nicotinic receptor expression by the ubiquitin-proteasome system.EMBO J. 2004 Oct 27;23(21):4156-65. doi: 10.1038/sj.emboj.7600436. Epub 2004 Oct 14. EMBO J. 2004. PMID: 15483627 Free PMC article.
-
Expression of gamma-aminobutyric acid rho 1 and rho 1 Delta 450 as gene fusions with the green fluorescent protein.Proc Natl Acad Sci U S A. 2001 Feb 13;98(4):1947-51. doi: 10.1073/pnas.98.4.1947. Epub 2001 Feb 6. Proc Natl Acad Sci U S A. 2001. PMID: 11172056 Free PMC article.
-
A conserved Cys-loop receptor aspartate residue in the M3-M4 cytoplasmic loop is required for GABAA receptor assembly.J Biol Chem. 2008 Oct 31;283(44):29740-52. doi: 10.1074/jbc.M802856200. Epub 2008 Aug 21. J Biol Chem. 2008. PMID: 18723504 Free PMC article.
-
Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons.J Neurosci. 2000 Nov 1;20(21):7972-7. doi: 10.1523/JNEUROSCI.20-21-07972.2000. J Neurosci. 2000. PMID: 11050117 Free PMC article.
References
-
- Backus KH, Arigoni M, Drescher U, Scheurer L, Malherbe P, Mohler H, Benson JA. Stoichiometry of a recombinant GABAA receptor deduced from mutation-induced rectification. NeuroReport. 1993;5:285–288. - PubMed
-
- Blair LA, Levitan ES, Marshall J, Dionne VE, Barnard EA. Single subunits of the GABAA receptor form ion channels with properties of the native receptor. Science. 1988;242:577–579. - PubMed
-
- Bogler O, Entwistle A, Kuhn R, Monuki E, Lemke G, Noble M. Single cell analysis of the expression of a nuclear-protein SCIP, by confocal microscopy. Histochem J. 1993;25:746–761. - PubMed
-
- Buller AL, Hastings GA, Kirkness EF, Fraser CM. Site-directed mutagenesis of N-linked glycosylation sites on the gamma-aminobutyric acid type A receptor alpha 1 subunit. Mol Pharmacol. 1994;46:858–865. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
