Subunit composition, kinetic, and permeation properties of AMPA receptors in single neocortical nonpyramidal cells
- PMID: 9254681
- PMCID: PMC6573153
- DOI: 10.1523/JNEUROSCI.17-17-06685.1997
Subunit composition, kinetic, and permeation properties of AMPA receptors in single neocortical nonpyramidal cells
Abstract
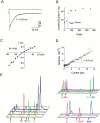
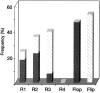
Native AMPA receptors (AMPARs) were investigated in neocortical fast-spiking (FS) and regular-spiking nonpyramidal (RSNP) cells. The onset of and recovery from desensitization as well as current rectification and single-channel conductance were studied by using fast glutamate application to outside-out patches. The GluR1-4 subunit, flip/flop splicing, and R/G editing expression patterns of functionally characterized cells were determined by single-cell reverse transcription-PCR to correlate the subunit composition of native AMPARs with their functional properties. Our sample, mostly constituted by RSNP neurons, predominantly expressed GluR3 flip and GluR2 flop. In individual cells, flip/flop splicing of each subunit appeared to be regulated independently, whereas for R/G editing all subunits were either almost fully edited or unedited. We confirmed that the relative GluR2 expression controls the permeation properties of native AMPARs, whereas none of the single molecular parameters considered appeared to be a key determinant of the kinetics. FS neurons displayed AMPARs with relatively homogeneous functional properties characterized by fast desensitization, slow recovery from desensitization, marked inward rectification, and large single-channel conductance. In contrast, these parameters varied over a wide range in RSNP neurons, and their combination resulted in various AMPAR functional patterns. Indeed, in different cells, fast or slow desensitization was found to be associated with either slow or fast recovery from desensitization. Similarly, fast or slow kinetics was associated with either strong or weak rectification. Our results suggest that kinetic and permeation properties of native AMPARs can be regulated independently in cortical neurons and probably do not have the same molecular determinants.
Figures




References
-
- Bochet P, Audinat E, Lambolez B, Crépel F, Rossier J, Lino M, Tsuzuki K, Ozawa S. Subunit composition at the single-cell level explains functional properties of a glutamate-gated channel. Neuron. 1994;12:383–388. - PubMed
-
- Bowie D, Mayer ML. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron. 1995;15:453–462. - PubMed
-
- Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 1990;13:99–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
