Cellular-synaptic generation of sleep spindles, spike-and-wave discharges, and evoked thalamocortical responses in the neocortex of the rat
- PMID: 9254689
- PMCID: PMC6573130
- DOI: 10.1523/JNEUROSCI.17-17-06783.1997
Cellular-synaptic generation of sleep spindles, spike-and-wave discharges, and evoked thalamocortical responses in the neocortex of the rat
Abstract
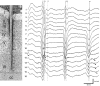
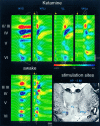
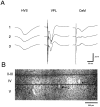
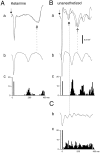
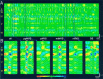
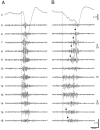
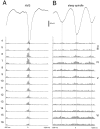
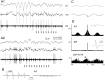
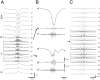
Thalamocortical neuronal oscillations underlie various field potentials that are expressed in the neocortex, including sleep spindles and high voltage spike-and-wave patterns (HVSs). The mechanism of extracellular current generation in the neocortex was studied in the anesthetized and awake rat. Field potentials and unit activity were recorded simultaneously along trajectories perpendicular to the cortical layers at spatial intervals of 100 microm by multiple-site recording silicon probes. Current source density (CSD) analysis revealed that the spatial positions of sinks in layers IV, V-VI, and II-III and of the accompanying sources were similar during sleep spindles, HVSs, and thalamic-evoked responses, although their relative strengths and timings differed. The magnitude and relative timing of the multiple pairs of sinks and sources determined the amplitude variability of HVSs and sleep spindles. The presence of temporally shifted dipoles was also supported by the time distribution of unit discharges in different layers. Putative interneurons discharged with repetitive bursts of 300-500 Hz. The spike component of HVSs was associated with fast field oscillations (400-600 Hz "ripples"). Discharges of pyramidal cells were phase-locked to the ripples. These findings indicate that the major extracellular currents underlying sleep spindles, HVSs, and evoked responses result from activation of intracortical circuitries. We hypothesize that the fast field ripples reflect summed IPSPs in pyramidal cells resulting from the high frequency barrage of interneurons.
Figures


References
-
- Andersen P, Andersson SA. Physiological basis of the alpha rhythm (Towe A, ed). Appleton-Century-Crofts; New York: 1968.
-
- Bal T, von Krosigk M, McCormick DA. From cellular to network mechanisms of a thalamic synchronized oscillation. In: Buzsáki G, Llinás R, Singer W, Berthoz A, Christen Y, editors. Temporal coding in the brain. Springer; Berlin: 1994. pp. 129–143.
-
- Bode-Greuel KM, Singer W, Aldenhoff JB. A current source density analysis of field potentials evoked in slices of visual cortex. Exp Brain Res. 1987;69:213–219. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
