The response of subthalamic nucleus neurons to dopamine receptor stimulation in a rodent model of Parkinson's disease
- PMID: 9254691
- PMCID: PMC6573155
- DOI: 10.1523/JNEUROSCI.17-17-06807.1997
The response of subthalamic nucleus neurons to dopamine receptor stimulation in a rodent model of Parkinson's disease
Abstract
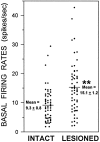
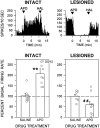
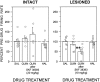
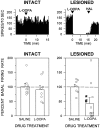
Overactivity in the subthalamic nucleus (STN) is believed to contribute to the pathophysiology of Parkinson's disease. It is hypothesized that dopamine receptor agonists reduce neuronal output from the STN. The present study tests this hypothesis by using in vivo extracellular single unit recording techniques to measure neuronal activity in the STN of rats with 6-hydroxydopamine-induced lesions of the nigrostriatal pathway (a model of Parkinson's disease). As predicted, firing rates of STN neurons in lesioned rats were tonically elevated under basal conditions and were decreased by the nonselective dopamine receptor agonists apomorphine and L-3, 4-dihydroxyphenylalanine (L-DOPA). STN firing rates were also decreased by the D2 receptor agonist quinpirole when administered after the D1 receptor agonist (+/-)- 1-phenyl-2,3,4,5-tetrahydro-(1H)-3-benzazepine-7,8-diol (SKF 38393). Results of the present study challenge the prediction that dopaminergic agonists reduce STN activity predominantly through actions at striatal dopamine D2 receptors. Firing rates of STN neurons were not altered by selective stimulation of D2 receptors and were increased by selective stimulation of D1 receptors. Moreover, there was a striking difference between the responses of the STN to D1/D2 receptor stimulation in the lesioned and intact rat; apomorphine inhibited STN firing in the lesioned rat and increased STN firing in the intact rat. These findings support the premise that therapeutic efficacy in the treatment of Parkinson's disease is associated with a decrease in the activity of the STN, but challenge assumptions about the roles of D1 and D2 receptors in the regulation of neuronal activity of the STN in both the intact and dopamine-depleted states.
Figures


Similar articles
-
Subthalamic nucleus lesions alter basal and dopamine agonist stimulated electrophysiological output from the rat basal ganglia.Synapse. 2004 Nov;54(2):119-28. doi: 10.1002/syn.20064. Synapse. 2004. PMID: 15352137
-
Effects of intrasubthalamic injection of dopamine receptor agonists on subthalamic neurons in normal and 6-hydroxydopamine-lesioned rats: an electrophysiological and c-Fos study.Neuroscience. 1999;92(2):533-43. doi: 10.1016/s0306-4522(98)00765-9. Neuroscience. 1999. PMID: 10408602
-
Effects of D1 and D2 dopamine receptor stimulation on the activity of substantia nigra pars reticulata neurons in 6-hydroxydopamine lesioned rats: D1/D2 coactivation induces potentiated responses.Brain Res. 1987 Mar 10;405(2):234-46. doi: 10.1016/0006-8993(87)90293-9. Brain Res. 1987. PMID: 2952219
-
Prior D1 dopamine receptor stimulation is required to prime D2-mediated striatal Fos expression in 6-hydroxydopamine-lesioned rats.Neuroscience. 1999;94(2):505-14. doi: 10.1016/s0306-4522(99)00338-3. Neuroscience. 1999. PMID: 10579212
-
The immunoregulatory role of dopamine: an update.Brain Behav Immun. 2010 May;24(4):525-8. doi: 10.1016/j.bbi.2009.10.015. Epub 2009 Nov 5. Brain Behav Immun. 2010. PMID: 19896530 Free PMC article. Review.
Cited by
-
Nicotinic receptor subtype-selective circuit patterns in the subthalamic nucleus.J Neurosci. 2015 Mar 4;35(9):3734-46. doi: 10.1523/JNEUROSCI.3528-14.2015. J Neurosci. 2015. PMID: 25740504 Free PMC article.
-
Role of external pallidal segment in primate parkinsonism: comparison of the effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism and lesions of the external pallidal segment.J Neurosci. 2004 Jul 21;24(29):6417-26. doi: 10.1523/JNEUROSCI.0836-04.2004. J Neurosci. 2004. PMID: 15269251 Free PMC article.
-
Low frequency stimulation of the pedunculopontine nucleus modulates electrical activity of subthalamic neurons in the rat.J Neural Transm (Vienna). 2009 Jan;116(1):51-6. doi: 10.1007/s00702-008-0155-z. Epub 2008 Nov 26. J Neural Transm (Vienna). 2009. PMID: 19034381
-
A Population Model of Deep Brain Stimulation in Movement Disorders From Circuits to Cells.Front Hum Neurosci. 2020 Mar 5;14:55. doi: 10.3389/fnhum.2020.00055. eCollection 2020. Front Hum Neurosci. 2020. PMID: 32210779 Free PMC article.
-
Push-pull effects of basal ganglia network in Parkinson's disease inferred by functional MRI.NPJ Parkinsons Dis. 2024 Nov 20;10(1):224. doi: 10.1038/s41531-024-00835-7. NPJ Parkinsons Dis. 2024. PMID: 39567536 Free PMC article.
References
-
- Abercrombie ED, Bonatz AE, Zigmond MJ. Effects of l-DOPA on extracellular dopamine in striatum of normal and 6-hydroxydopamine-treated rats. Brain Res. 1990;525:36–44. - PubMed
-
- Afsharpour S. Topographical projections of the cerebral cortex to the subthalamic nucleus. J Comp Neurol. 1985;236:14–28. - PubMed
-
- Albin RL, Young AB, Penney JP. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. - PubMed
-
- Allers KA, Kreiss DS, Twery MJ, Juncos JL, Walters JR. The role of glutamate in D1 agonist induced increases of subthalamic nucleus neuronal firing rate: normal versus 6-OHDA lesioned rats. Soc Neurosci Abstr. 1996;22:1200.
-
- Anderson JJ, Chase TN, Engber TM. Differential effect of subthalamic nucleus ablation on dopamine D1 and D2 agonist-induced rotation in 6-hydroxydopamine-lesioned rats. Brain Res. 1992;588:307–310. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
