A protein encoded by a group I intron in Aspergillus nidulans directly assists RNA splicing and is a DNA endonuclease
- PMID: 9256423
- PMCID: PMC22997
- DOI: 10.1073/pnas.94.17.8994
A protein encoded by a group I intron in Aspergillus nidulans directly assists RNA splicing and is a DNA endonuclease
Erratum in
- Proc Natl Acad Sci U S A 1997 Dec 23;94(26):14976
Abstract
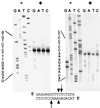
Some group I introns self-splice in vitro, but almost all are thought to be assisted by proteins in vivo. Mutational analysis has shown that the splicing of certain group I introns depends upon a maturase protein encoded by the intron itself. However the effect of a protein on splicing can be indirect. We now provide evidence that a mitochondrial intron-encoded protein from Aspergillus nidulans directly facilitates splicing in vitro. This demonstrates that a maturase is an RNA splicing protein. The protein-assisted reaction is as fast as that of any other known group I intron. Interestingly the protein is also a DNA endonuclease, an activity required for intron mobilization. Mobile elements frequently encode proteins that promote their propagation. Intron-encoded proteins that also assist RNA splicing would facilitate both the transposition and horizontal transmission of introns.
Figures


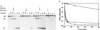


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

