The roles of the two proton input channels in cytochrome c oxidase from Rhodobacter sphaeroides probed by the effects of site-directed mutations on time-resolved electrogenic intraprotein proton transfer
- PMID: 9256439
- PMCID: PMC23042
- DOI: 10.1073/pnas.94.17.9085
The roles of the two proton input channels in cytochrome c oxidase from Rhodobacter sphaeroides probed by the effects of site-directed mutations on time-resolved electrogenic intraprotein proton transfer
Abstract
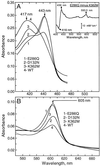
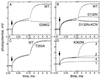
The crystal structures of cytochrome c oxidase from both bovine and Paracoccus denitrificans reveal two putative proton input channels that connect the heme-copper center, where dioxygen is reduced, to the internal aqueous phase. In this work we have examined the role of these two channels, looking at the effects of site-directed mutations of residues observed in each of the channels of the cytochrome c oxidase from Rhodobacter sphaeroides. A photoelectric technique was used to monitor the time-resolved electrogenic proton transfer steps associated with the photo-induced reduction of the ferryl-oxo form of heme a3 (Fe4+ = O2-) to the oxidized form (Fe3+OH-). This redox step requires the delivery of a "chemical" H+ to protonate the reduced oxygen atom and is also coupled to proton pumping. It is found that mutations in the K channel (K362M and T359A) have virtually no effect on the ferryl-oxo-to-oxidized (F-to-Ox) transition, although steady-state turnover is severely limited. In contrast, electrogenic proton transfer at this step is strongly suppressed by mutations in the D channel. The results strongly suggest that the functional roles of the two channels are not the separate delivery of chemical or pumped protons, as proposed recently [Iwata, S., Ostermeier, C., Ludwig, B. & Michel, H. (1995) Nature (London) 376, 660-669]. The D channel is likely to be involved in the uptake of both "chemical" and "pumped" protons in the F-to-Ox transition, whereas the K channel is probably idle at this partial reaction and is likely to be used for loading the enzyme with protons at some earlier steps of the catalytic cycle. This conclusion agrees with different redox states of heme a3 in the K362M and E286Q mutants under aerobic steady-state turnover conditions.
Figures


References
-
- Saraste M, Holm L, Lemieux L, Lubben M, van der Oost J. Biochem Soc Trans. 1991;19:608–612. - PubMed
-
- Babcock G T, Wikström M. Nature (London) 1992;356:301–309. - PubMed
-
- Iwata S, Ostermeier C, Ludwig B, Michel H. Nature (London) 1995;376:660–669. - PubMed
-
- Tsukihara T, Aoyama H, Yamashita E, Tomizaki T, Yamaguchi H, Shinzawa-Itoh K, Nakashima T, Yaono R, Yoshikawa S. Science. 1995;269:1069–1074. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

