mRNA binding protein mrnp 41 localizes to both nucleus and cytoplasm
- PMID: 9256445
- PMCID: PMC23064
- DOI: 10.1073/pnas.94.17.9119
mRNA binding protein mrnp 41 localizes to both nucleus and cytoplasm
Abstract
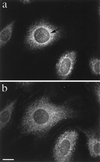
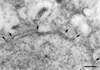
We have identified and molecularly characterized a human protein with a Mr of 40,880 Da. After UV irradiation of HeLa cells, this protein was cross-linked to poly(A)-containing mRNA and was therefore designated mrnp 41 (for mRNA binding protein of 41 kDa). Cell fractionation and immunoblotting showed mrnp 41 in both the cytoplasm and the nucleus and particularly in the nuclear envelope. Immunofluorescence microscopy localized mrnp 41 to distinct foci in the nucleoplasm, to the nuclear rim, and to meshwork-like structures throughout the cytoplasm. The cytoplasmic meshwork staining was disrupted by prior treatment of cells with the actin filament- or microtubule-disrupting drugs cytochalasin or nocodazole, respectively, suggesting association of mrnp 41 with the cytoskeleton. Double immunofluorescence with antibodies against mrnp 41 and the cytoplasmic poly(A) binding protein showed colocalization to the cytoplasmic meshwork. Immunogold electronmicroscopy confirmed mrnp 41's cytoplasmic and nucleoplasmic localization and revealed a striking labeling of nuclear pore complexes. Together these data suggest that mrnp 41 may function in nuclear export of mRNPs and/or in cytoplasmic transport on, or attachment to, the cytoskeleton. Consistent with a role of mrnp 41 in nuclear export are previous reports that mutations in homologs of mrnp 41 in Schizosaccharomyces pombe, designated Rae1p, or in Saccharomyces cerevisiae, designated Gle2p, result in mRNA accumulation in the nucleus although it is presently not known whether these homologs are mRNA binding proteins as well.
Figures




References
-
- Daneholt B. Cell. 1997;88:585–588. - PubMed
-
- Piñol-Roma S, Dreyfuss G. Trends Cell Biol. 1993;3:151–155. - PubMed
-
- Piñol-Roma S, Dreyfuss G. Nature (London) 1992;355:730–735. - PubMed
-
- Visa N, Alzhanova-Ericsson A T, Sun X, Kiseleva E, Björkroth B, Wurtz T, Daneholt B. Cell. 1996;84:253–264. - PubMed
-
- Görlach M, Burd C C, Dreyfuss G. Exp Cell Res. 1994;211:400–407. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

