The beta subunit of CKII negatively regulates Xenopus oocyte maturation
- PMID: 9256448
- PMCID: PMC23074
- DOI: 10.1073/pnas.94.17.9136
The beta subunit of CKII negatively regulates Xenopus oocyte maturation
Abstract
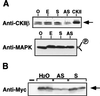
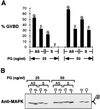
CKII (formerly known as casein kinase II) is a ubiquitously expressed enzyme that plays an important role in regulating cell growth and differentiation. The beta subunit of CKII (CKIIbeta) is not catalytic but forms heterotetramers with the catalytic subunit alpha to generate an alpha2beta2 holoenzyme. In Xenopus oocytes, CKIIbeta also associates with another serine/threonine kinase, Mos. As a key regulator of meiosis, Mos is necessary and sufficient to initiate oocyte maturation. We have previously shown that the binding of CKIIbeta to Mos represses Mos-mediated mitogen-activated protein kinase (MAPK) activation and that the ectopic expression of CKIIbeta inhibits progesterone-induced Xenopus oocyte maturation. We have now used an antisense oligonucleotide technique to reduce the endogenous CKIIbeta protein level in Xenopus oocytes, and we find that oocytes with a reduced content of CKIIbeta are more sensitive to low doses of progesterone and show accelerated MAPK activation and germinal vesicle breakdown. Furthermore, ectopic expression of a Mos-binding fragment of CKIIbeta suppressed the effect of antisense oligonucleotide. These results suggest that the endogenous CKIIbeta normally sets a threshold level for Mos protein, which must be exceeded for Mos to activate the MAPK signaling pathway and induce oocyte maturation.
Figures


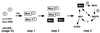
References
-
- Pinna L A. Biochim Biophys Acta. 1990;1054:267–284. - PubMed
-
- Krebs E G, Eisenman R N, Kuenzel E A, Litchfield D W, Lozeman F J, Luscher B, Sommercorn J. Cold Spring Harbor Symp Quant Biol. 1988;53:77–84. - PubMed
-
- Allende J E, Allende C C. FASEB J. 1995;9:313–323. - PubMed
-
- Gietz R D, Graham K C, Litchfield D W. J Biol Chem. 1995;270:13017–13021. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

