Expression of Sonic hedgehog gene in regenerating newt limb blastemas recapitulates that in developing limb buds
- PMID: 9256452
- PMCID: PMC23086
- DOI: 10.1073/pnas.94.17.9159
Expression of Sonic hedgehog gene in regenerating newt limb blastemas recapitulates that in developing limb buds
Abstract
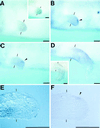
This study aimed at characterizing the Sonic hedgehog (shh) gene in newt limbs, which encodes a signaling molecule of the zone of polarizing activity (ZPA) responsible for determining the anterior-posterior axis of the embryonic chicken and mouse limbs. The reverse transcription-PCR showed that adult newt regenerating limbs express shh genes. In situ hybridization experiments demonstrated that shh genes were expressed in mesenchymal cells of the posterior region of both embryonic buds and regenerating blastemas of newt limbs, strongly suggesting the presence of ZPA in these tissues. Experiments of the axial reversal graft of blastemas further supported this suggestion. The grafted blastemas regenerated supernumerary limbs, and this has been explained by three models: the polar coordinate model, the boundary model, and the polarizing zone model. In favor of the third model, the shh gene was expressed not only in the original region (new anterior region) of the graft, but also ectopically in the other region (new posterior region) of the same graft. This study implies that the regenerating limb blastema produces ZPA as the signaling center of the AP patterning as in the developing limb bud and, therefore, supports the notion that the limb regeneration recapitulates the limb development.
Figures
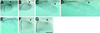


References
-
- Wallace H. Vertebrate Limb Regeneration. Toronto: Wiley; 1981.
-
- Stocum D L. Molecular Biology Intelligence Unit: Wound Repair, Regeneration and Artificial Tissues. New York: Springer; 1995.
-
- Tabin C J. Cell. 1991;66:199–217. - PubMed
-
- Riddle R D, Johnson R L, Laufer E, Tabin C. Cell. 1993;75:1401–1416. - PubMed
-
- Tickle C, Eichele G A. Rev Cell Biol. 1994;10:121–152. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

