The human XRCC9 gene corrects chromosomal instability and mutagen sensitivities in CHO UV40 cells
- PMID: 9256465
- PMCID: PMC23130
- DOI: 10.1073/pnas.94.17.9232
The human XRCC9 gene corrects chromosomal instability and mutagen sensitivities in CHO UV40 cells
Abstract
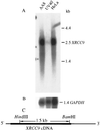
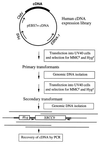
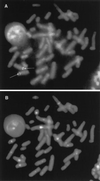
The Chinese hamster ovary (CHO) mutant UV40 cell line is hypersensitive to UV and ionizing radiation, simple alkylating agents, and DNA cross-linking agents. The mutant cells also have a high level of spontaneous chromosomal aberrations and 3-fold elevated sister chromatid exchange. We cloned and sequenced a human cDNA, designated XRCC9, that partially corrected the hypersensitivity of UV40 to mitomycin C, cisplatin, ethyl methanesulfonate, UV, and gamma-radiation. The spontaneous chromosomal aberrations in XRCC9 cDNA transformants were almost fully corrected whereas sister chromatid exchanges were unchanged. The XRCC9 genomic sequence was cloned and mapped to chromosome 9p13. The translated XRCC9 sequence of 622 amino acids has no similarity with known proteins. The 2.5-kb XRCC9 mRNA seen in the parental cells was undetectable in UV40 cells. The mRNA levels in testis were up to 10-fold higher compared with other human tissues and up to 100-fold higher compared with other baboon tissues. XRCC9 is a candidate tumor suppressor gene that might operate in a postreplication repair or a cell cycle checkpoint function.
Figures



References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

