Interaction of CTLA-4 with AP50, a clathrin-coated pit adaptor protein
- PMID: 9256472
- PMCID: PMC23153
- DOI: 10.1073/pnas.94.17.9273
Interaction of CTLA-4 with AP50, a clathrin-coated pit adaptor protein
Abstract
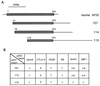
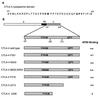
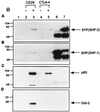
CTLA-4 plays a critical role in regulating the immune response. It is mainly located in cytoplasmic vesicles and is expressed only transiently on the surface after T cell activation. In this study, we demonstrate that CTLA-4 is associated with AP50, the medium chain of the clathrin-associated coated pit adaptor protein complex AP2. In a yeast two-hybrid screen, three individual cDNA clones that encode mouse AP50 were isolated, all of which can interact specifically with the cytoplasmic domain of mouse CTLA-4, but not with the cytoplasmic domain of mouse CD28. We have shown that CTLA-4 can bind specifically to AP50 when CTLA-4 and AP50 are cotransfected into human 293T cells. A Y201 to F201 mutation in the YVKM intracellular localization motif of the CTLA-4 cytoplasmic domain significantly diminished its binding to AP50. We also found that AP50 bound to a CTLA-4 peptide containing unphosphorylated Y201 but not to a peptide containing phosphorylated Y201. Conversely, the p85 subunit of phosphatidylinositol 3-kinase and, to a lesser extent, protein tyrosine phosphatase SYP (SHP-2) and SHP (SHP-1) bind only to the CTLA-4 peptide containing phosphorylated Y201. Therefore, the phosphorylation status of Y201 in the CTLA-4 cytoplasmic domain determines the binding specificity of CTLA-4. These results suggest that AP50 and the coated pit adaptor complex AP2 may play an important role in regulating the intracellular trafficking and function of CTLA-4.
Figures
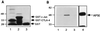


References
-
- Linsley P S, Ledbetter J A. Annu Rev Immunol. 1993;11:191–212. - PubMed
-
- June C H, Bluestone J A, Nadler L M, Thompson C B. Immunol Today. 1994;15:321–331. - PubMed
-
- Allison J P. Curr Opin Immunol. 1994;6:414–419. - PubMed
-
- Brunet J F, Denizot F, Luciani M F, Roux-Dosseto M, Suzan M, Mattei M F, Golstein P. Nature (London) 1987;328:267–270. - PubMed
-
- Harper K, Balzano C, Rouvier E, Mattei M G, Luciani M F, Golstein P. J Immunol. 1991;147:1037–1044. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

