Potent, protective anti-HIV immune responses generated by bimodal HIV envelope DNA plus protein vaccination
- PMID: 9256490
- PMCID: PMC23198
- DOI: 10.1073/pnas.94.17.9378
Potent, protective anti-HIV immune responses generated by bimodal HIV envelope DNA plus protein vaccination
Abstract
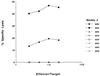
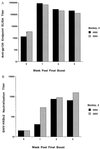
It is generally thought that an effective vaccine to prevent HIV-1 infection should elicit both strong neutralizing antibody and cytotoxic T lymphocyte responses. We recently demonstrated that potent, boostable, long-lived HIV-1 envelope (Env)-specific cytotoxic T lymphocyte responses can be elicited in rhesus monkeys using plasmid-encoded HIV-1 env DNA as the immunogen. In the present study, we show that the addition of HIV-1 Env protein to this regimen as a boosting immunogen generates a high titer neutralizing antibody response in this nonhuman primate species. Moreover, we demonstrate in a pilot study that immunization with HIV-1 env DNA (multiple doses) followed by a final immunization with HIV-1 env DNA plus HIV-1 Env protein (env gene from HXBc2 clone of HIV IIIB; Env protein from parental HIV IIIB) completely protects monkeys from infection after i.v. challenge with a chimeric virus expressing HIV-1 env (HXBc2) on a simian immmunodeficiency virusmac backbone (SHIV-HXBc2). The potent immunity and protection seen in these pilot experiments suggest that a DNA prime/DNA plus protein boost regimen warrants active investigation as a vaccine strategy to prevent HIV-1 infection.
Figures



References
-
- Johnston M I. Int Arch Allergy Immunol. 1995;108:313–317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

