Essential role of POU-domain factor Brn-3c in auditory and vestibular hair cell development
- PMID: 9256502
- PMCID: PMC23217
- DOI: 10.1073/pnas.94.17.9445
Essential role of POU-domain factor Brn-3c in auditory and vestibular hair cell development
Abstract
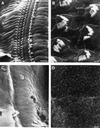
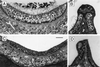
The Brn-3 subfamily of POU-domain transcription factor genes consists of three highly homologous members-Brn-3a, Brn-3b, and Brn-3c-that are expressed in sensory neurons and in a small number of brainstem nuclei. This paper describes the role of Brn-3c in auditory and vestibular system development. In the inner ear, the Brn-3c protein is found only in auditory and vestibular hair cells, and the Brn-3a and Brn-3b proteins are found only in subsets of spiral and vestibular ganglion neurons. Mice carrying a targeted deletion of the Brn-3c gene are deaf and have impaired balance. These defects reflect a complete loss of auditory and vestibular hair cells during the late embryonic and early postnatal period and a secondary loss of spiral and vestibular ganglion neurons. Together with earlier work demonstrating a loss of trigeminal ganglion neurons and retinal ganglion cells in mice carrying targeted disruptions in the Brn-3a and Brn-3b genes, respectively, the Brn-3c phenotype reported here demonstrates that each of the Brn-3 genes plays distinctive roles in the somatosensory, visual, and auditory/vestibular systems.
Figures






References
-
- Jan Y N, Jan L. Cell. 1993;75:827–830. - PubMed
-
- Lee J E, Hollenberg S M, Snider L, Turner D L, Lipnick N, Weintraub H. Science. 1995;268:836–844. - PubMed
-
- Keynes R, Krumlauf R. Annu Rev Neurosci. 1994;17:109–132. - PubMed
-
- Ryan A K, Rosenfeld M G. Genes Dev. 1997;11:1207–1225. - PubMed
-
- Herr W, Sturm R A, Clerc R G, Corcoran L M, Baltimore D, Sharp P A, Ingraham H A, Rosenfeld M G, Finney M, Ruvkin G, Horvitz H R. Genes Dev. 1988;2:1513–1516. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

