Myofibrillogenesis visualized in living embryonic cardiomyocytes
- PMID: 9256510
- PMCID: PMC23235
- DOI: 10.1073/pnas.94.17.9493
Myofibrillogenesis visualized in living embryonic cardiomyocytes
Abstract
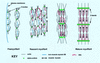
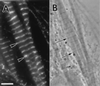
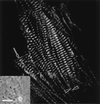
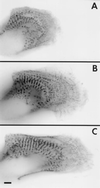
Myofibril formation was visualized in cultured live cardiomyocytes that were transfected with plasmids expressing green fluorescent protein (GFP) linked to the Z-band protein, alpha-actinin. The expression of this fluorescent protein provided an in vivo label for structures containing alpha-actinin. The GFP-alpha-actinin fusion protein was incorporated into Z-bands, intercalated discs, and attachment plaques, as well as into the punctate aggregates, or Z-bodies, that are thought to be the precursors of Z-bands. Observations of live cells over several days in culture permitted us to test aspects of several theories of myofibril assembly that had been proposed previously based on the study of fixed cells. Fine fibrils, called premyofibrils, that formed de novo at the spreading edges of cardiomyocytes, contained punctate concentrations of alpha-actinin, termed Z-bodies. The punctate Z-bodies grew and aligned with Z-bodies in adjacent fibrils. With increasing time, adjacent fibrils and Z-bodies appeared to fuse and form mature myofibrils and Z-bands in cytoplasmic regions where the linear arrays of Z-bodies had been. These new myofibrils became aligned with existing myofibrils at their Z-bands to form myofibrils that spanned the length of the spread cell. These results are consistent with a model that postulates that the fibrils that form de novo near the cell membrane are premyofibrils-i.e., the precursors of mature myofibrils.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

