Structure-activity relationships of 4-(phenylethynyl)-6-phenyl-1,4-dihydropyridines as highly selective A3 adenosine receptor antagonists
- PMID: 9258367
- PMCID: PMC10788687
- DOI: 10.1021/jm970091j
Structure-activity relationships of 4-(phenylethynyl)-6-phenyl-1,4-dihydropyridines as highly selective A3 adenosine receptor antagonists
Abstract
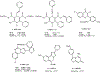
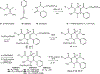
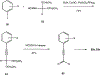
4-(Phenylethynyl)-6-phenyl-1,4-dihydropyridine derivatives are selective antagonists at human A3 adenosine receptors, with Ki values in a radioligand binding assay vs [125I]AB-MECA (N6-(4-amino-3-iodobenzyl)-5'-(N-methylcarbamoyl)adenosine) in the submicromolar range. In this study, structure-activity relationships at various positions of the dihydropyridine ring (the 3- and 5-acyl substituents, the 4-aryl substituent, and 1-methyl group) were probed synthetically. Using the combined protection of the 1-ethoxymethyl and the 5-[2-(trimethylsilyl)ethyl] ester groups, a free carboxylic acid was formed at the 5-position allowing various substitutions. Selectivity of the new analogues for cloned human A3 adenosine receptors was determined vs radioligand binding at rat brain A1 and A2A receptors. Structure-activity analysis at adenosine receptors indicated that pyridyl, furyl, benzofuryl, and thienyl groups at the 4-position resulted in, at most, only moderate selectivity for A3 adenosine receptors. Ring substitution (e.g., 4-nitro) of the 4-phenylethylnyl group did not provide enhanced selectivity, as it did for the 4-styryl-substituted dihydropyridines. At the 3-position of the dihydropyridine ring, esters were much more selective for A3 receptors than closely related thioester, amide, and ketone derivatives. A cyclic 3-keto derivative was 5-fold more potent at A3 receptors than a related open-ring analogue. At the 5-position, a homologous series of phenylalkyl esters and a series of substituted benzyl esters were prepared and tested. (Trifluoromethyl)-, nitro-, and other benzyl esters substituted with electron-withdrawing groups were specific for A3 receptors with nanomolar Ki values and selectivity as high as 37000-fold. A functionalized congener bearing an [(aminoethyl)amino]carbonyl group was also prepared as an intermediate in the synthesis of biologically active conjugates.
Figures

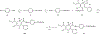
References
-
- Ramkumar V; Stiles GL; Beaven MA; Ali H. The A3 adenosine receptor is the unique adenosine receptor which facilitates release of allergic mediators in mast cells. J. Biol. Chem 1993, 268, 16887–16890. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

