A new function for the LDL receptor: transcytosis of LDL across the blood-brain barrier
- PMID: 9265653
- PMCID: PMC2138047
- DOI: 10.1083/jcb.138.4.877
A new function for the LDL receptor: transcytosis of LDL across the blood-brain barrier
Abstract
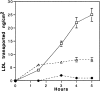
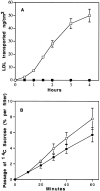
Lipoprotein transport across the blood-brain barrier (BBB) is of critical importance for the delivery of essential lipids to the brain cells. The occurrence of a low density lipoprotein (LDL) receptor on the BBB has recently been demonstrated. To examine further the function of this receptor, we have shown using an in vitro model of the BBB, that in contrast to acetylated LDL, which does not cross the BBB, LDL is specifically transcytosed across the monolayer. The C7 monoclonal antibody, known to interact with the LDL receptor-binding domain, totally blocked the transcytosis of LDL, suggesting that the transcytosis is mediated by the receptor. Furthermore, we have shown that cholesterol-depleted astrocytes upregulate the expression of the LDL receptor at the BBB. Under these conditions, we observed that the LDL transcytosis parallels the increase in the LDL receptor, indicating once more that the LDL is transcytosed by a receptor-mediated mechanism. The nondegradation of the LDL during the transcytosis indicates that the transcytotic pathway in brain capillary endothelial cells is different from the LDL receptor classical pathway. The switch between a recycling receptor to a transcytotic receptor cannot be explained by a modification of the internalization signals of the cytoplasmic domain of the receptor, since we have shown that LDL receptor messengers in growing brain capillary ECs (recycling LDL receptor) or differentiated cells (transcytotic receptor) are 100% identical, but we cannot exclude posttranslational modifications of the cytoplasmic domain, as demonstrated for the polymeric immunoglobulin receptor. Preliminary studies suggest that caveolae are likely to be involved in the potential transport of LDL from the blood to the brain.
Figures







References
-
- Albers JJ, Tollefson JH, Wolfbauer G, Albright RE. Cholesteryl ester transfer protein in human brain. Int J Clin Lab Res. 1992;21:264–266. - PubMed
-
- Anderson RGW. Plasmalemmal caveolae and GPI-anchored membrane proteins. Curr Opin Cell Biol. 1993;5:647–652. - PubMed
-
- Beisiegel U, Schneider WJ, Goldstein JL, Anderson RGW, Brown MS. Monoclonal antibodies to the low-density lipoprotein receptor as a probe to study of receptor mediated endocytosis and the genetics of familial hypercholesterolemia. J Biol Chem. 1981;256:11923–11931. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

