COOH terminus of occludin is required for tight junction barrier function in early Xenopus embryos
- PMID: 9265654
- PMCID: PMC2138038
- DOI: 10.1083/jcb.138.4.891
COOH terminus of occludin is required for tight junction barrier function in early Xenopus embryos
Abstract
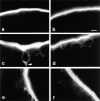
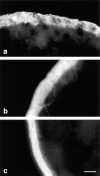
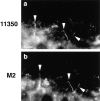
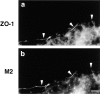
Occludin is the only known integral membrane protein localized at the points of membrane- membrane interaction of the tight junction. We have used the Xenopus embryo as an assay system to examine: (a) whether the expression of mutant occludin in embryos will disrupt the barrier function of tight junctions, and (b) whether there are signals within the occludin structure that are required for targeting to the sites of junctional interaction. mRNAs transcribed from a series of COOH-terminally truncated occludin mutants were microinjected into the antero-dorsal blastomere of eight-cell embryos. 8 h after injection, the full-length and the five COOH-terminally truncated proteins were all detected at tight junctions as defined by colocalization with both endogenous occludin and zonula occludens-1 demonstrating that exogenous occludin correctly targeted to the tight junction. Importantly, our data show that tight junctions containing four of the COOH-terminally truncated occludin proteins were leaky; the intercellular spaces between the apical cells were penetrated by sulfosuccinimidyl-6-(biotinamido) Hexanoate (NHS-LC-biotin). In contrast, embryos injected with mRNAs coding for the full-length, the least truncated, or the soluble COOH terminus remained impermeable to the NHS-LC-biotin tracer. The leakage induced by the mutant occludins could be rescued by coinjection with full-length occludin mRNA. Immunoprecipitation analysis of detergent-solubilized embryo membranes revealed that the exogenous occludin was bound to endogenous Xenopus occludin in vivo, indicating that occludin oligomerized during tight junction assembly. Our data demonstrate that the COOH terminus of occludin is required for the correct assembly of tight junction barrier function. We also provide evidence for the first time that occludin forms oligomers during the normal process of tight junction assembly. Our data suggest that mutant occludins target to the tight junction by virtue of their ability to oligomerize with full-length endogenous molecules.
Figures



References
-
- Balda MS, Whitney JA, Flores C, Gonzalez S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996;134:1031–1049. - PMC - PubMed
-
- Cereijido M, Ponce A, Gonzalez-Mariscal L. Tight junctions and apical/basolateral polarity. J Membr Biol. 1989;110:1–9.
-
- Citi S, Sabanay H, Jakes R, Geiger B, Kendrick-Jones J. Cingulin, a new peripheral component of tight junctions. Nature (Lond) 1988;333:272–276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

