Fidelity and mutational spectrum of Pfu DNA polymerase on a human mitochondrial DNA sequence
- PMID: 9267808
- PMCID: PMC310667
- DOI: 10.1101/gr.7.8.843
Fidelity and mutational spectrum of Pfu DNA polymerase on a human mitochondrial DNA sequence
Abstract
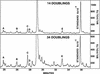
The study of rare genetic changes in human tissues requires specialized techniques. Point mutations at fractions at or below 10(-6) must be observed to discover even the most prominent features of the point mutational spectrum. PCR permits the increase in number of mutant copies but does so at the expense of creating many additional mutations or "PCR noise". Thus, each DNA sequence studied must be characterized with regard to the DNA polymerase and conditions used to avoid interpreting a PCR-generated mutation as one arising in human tissue. The thermostable DNA polymerase derived from Pyrococcus furiosus designated Pfu has the highest fidelity of any DNA thermostable polymerase studied to date, and this property recommends it for analyses of tissue mutational spectra. Here, we apply constant denaturant capillary electrophoresis (CDCE) to separate and isolate the products of DNA amplification. This new strategy permitted direct enumeration and identification of point mutations created by Pfu DNA polymerase in a 96-bp low melting domain of a human mitochondrial sequence despite the very low mutant fractions generated in the PCR process. This sequence, containing part of the tRNA glycine and NADH dehydrogenase subunit 3 genes, is the target of our studies of mitochondrial mutagenesis in human cells and tissues. Incorrectly synthesized sequences were separated from the wild type as mutant/wild-type heteroduplexes by sequential enrichment on CDCE. An artificially constructed mutant was used as an internal standard to permit calculation of the mutant fraction. Our study found that the average error rate (mutations per base pair duplication) of Pfu was 6.5 x 10(-7), and five of its more frequent mutations (hot spots) consisted of three transversions (GC-->TA, AT-->TA, and AT-->CG), one transition (AT-->GC), and one 1-bp deletion (in an AAAAAA sequence). To achieve an even higher sensitivity, the amount of Pfu-induced mutants must be reduced.
Figures

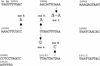
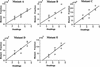
References
-
- Barnes WM. The fidelity of Taq polymerase catalyzing PCR is improved by an N-terminal deletion. Gene. 1992;112:29–35. - PubMed
-
- Brail L, Fan E, Levin DB, Logan DM. Improved polymerase fidelity in PCR-SSCPA. Mutat Res. 1993;303:171–175. - PubMed
-
- Cariello NF, Skopek TR. Mutational analysis using denaturing gradient gel electrophoresis and PCR. Mutat Res. 1993;288:103–112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
