The response to extracytoplasmic stress in Escherichia coli is controlled by partially overlapping pathways
- PMID: 9271123
- PMCID: PMC316410
- DOI: 10.1101/gad.11.15.2012
The response to extracytoplasmic stress in Escherichia coli is controlled by partially overlapping pathways
Abstract
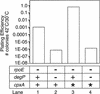
The activity of the alternate sigma-factor sigmaE of Escherichia coli is induced by several stressors that lead to the extracytoplasmic accumulation of misfolded or unfolded protein. The sigmaE regulon contains several genes, including that encoding the periplasmic protease DegP, whose products are thought to be required for maintaining the integrity of the cell envelope because cells lacking sigmaE are sensitive to elevated temperature and hydrophobic agents. Selection of multicopy suppressors of the temperature-sensitive phenotype of cells lacking sigmaE revealed that overexpression of the lipoprotein NlpE restored high temperature growth to these cells. Overexpression of NlpE has been shown previously to induce DegP synthesis by activating the Cpx two-component signal transduction pathway, and suppression of the temperature-sensitive phenotype by NlpE was found to be dependent on the Cpx proteins. In addition, a constitutively active form of the CpxA sensor/kinase also fully suppressed the temperature-sensitive defect of cells lacking sigmaE. DegP was found to be necessary, but not sufficient, for suppression. Activation of the Cpx pathway has also been shown to alleviate the toxicity of several LamB mutant proteins. Together, these results reveal the existence of two partially overlapping regulatory systems involved in the response to extracytoplasmic stress in E. coli.
Figures





References
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Bartolomé B, Jubete Y, Martinez E, de la Cruz F. Construction and properties of a family of pACYC184-derived cloning vectors compatible with pBR322 and its derivatives. Gene. 1991;102:75–78. - PubMed
-
- Becker J, Craig EA. Heat-shock proteins as molecular chaperones. Eur J Biochem. 1994;219:11–23. - PubMed
-
- Bolivar F, Rodriguez RL, Greene PJ, Betlach MC, Heyneker HL, Boyer HW. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2:95–113. - PubMed
-
- Bukau B. Regulation of the Escherichia coli heat-shock response. Mol Microbiol. 1993;9:671–680. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
