Impaired inflammatory responses in the reverse arthus reaction through genetic deletion of the C5a receptor
- PMID: 9271590
- PMCID: PMC2199021
- DOI: 10.1084/jem.186.5.749
Impaired inflammatory responses in the reverse arthus reaction through genetic deletion of the C5a receptor
Abstract
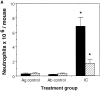
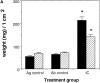
We recently demonstrated that gene-targeted disruption of the C5a anaphylatoxin receptor prevented lung injury in immune complex-mediated inflammation. In this study, we compare the effect of C5aR deficiency in immune complex-induced inflammation in the peritoneal cavity and skin with the results derived from our immune complex alveolitis model. C5aR- deficient mice exhibit decreased migration of neutrophils and decreased levels of TNF-alpha and interleukin 6 in the peritoneal reverse passive Arthus reaction compared to their wild-type littermates. In the reverse passive Arthus reaction in the skin the C5aR was also required for the full expression of neutrophil influx and edema formation; C5aR-deficient mice showed reduced neutrophil migration and microvascular permeability changes. In contrast to our studies in immune complex-induced lung inflammation, C5aR deficiency does not completely prevent injury in the peritoneal cavity and skin. These data indicate a dominant role for the C5aR and its ligand in the reverse passive Arthus reaction in the lung and a synergistic role together with other inflammatory mediators in immune complex-mediated peritonitis and skin injury.
Figures






References
-
- Arthus M. Injections répétées de sérum de cheval chez le lapin. Soc Biol. 1903;55:817–825.
-
- Cochrane, C.G., and A. Janoff. 1974. Chapter 3. In The Inflammatory Process, Vol. 3. B.W. Zweifach, L. Grant, and R.T. McCluskey, editors. Academic Press, New York.
-
- Gerard C, Gerard NP. C5a anaphylatoxin and its seven transmembrane-segment receptor. Annu Rev Immunol. 1994;12:775–808. - PubMed
-
- Goldstein, I.M. 1988. Complement: biologically active products. In Inflammation: Basic Principles and Clinical Correlates. J.I. Gallin, I.M. Goldstein, and R. Snyderman, editors. Raven Press, New York. 55.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

