Changes of telomere length cause reciprocal changes in the lifespan of mother cells in Saccharomyces cerevisiae
- PMID: 9275199
- PMCID: PMC23265
- DOI: 10.1073/pnas.94.18.9768
Changes of telomere length cause reciprocal changes in the lifespan of mother cells in Saccharomyces cerevisiae
Abstract
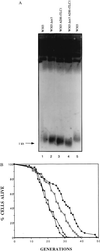
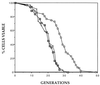
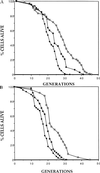
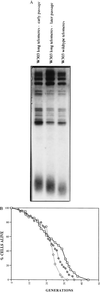
Budding yeast cells divide asymmetrically, giving rise to a mother and its daughter. Mother cells have a limited division potential, called their lifespan, which ends in proliferation-arrest and lysis. In this report we mutate telomerase in Saccharomyces cerevisiae to shorten telomeres and show that, rather than shortening lifespan, this leads to a significant extension in lifespan. This extension requires the product of the SIR3 gene, an essential component of the silencing machinery which binds to telomeres. In contrast, longer telomeres in a genotypically wild-type strain lead to a decrease in lifespan. These findings suggest that the length of telomeres dictates the lifespan by regulating the amount of the silencing machinery available to nontelomeric locations in the yeast genome.
Figures

References
-
- Harley C B, Futcher A B, Greider C W. Nature (London) 1990;345:458–460. - PubMed
-
- Hastie N D, Dempster M, Dunlop M G, Thompson A M, Green D K, Allshire R C. Nature (London) 1990;346:866–868. - PubMed
-
- Greider C W, Harley C B. In: Cellular Aging and Cell Death. Holbrook N J, Martin G R, Lockshin R A, editors. New York: Wiley–Liss; 1996. pp. 123–138.
-
- Kim N W, Piatyszek M A, Prowse K R, Harley C B, West M D, Ho P L, Coviello G M, Wright W E, Weinrich S L, Shay J W. Science. 1994;266:2011–2014. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

