Neuronal nicotinic threonine-for-leucine 247 alpha7 mutant receptors show different gating kinetics when activated by acetylcholine or by the noncompetitive agonist 5-hydroxytryptamine
- PMID: 9275226
- PMCID: PMC23293
- DOI: 10.1073/pnas.94.18.9915
Neuronal nicotinic threonine-for-leucine 247 alpha7 mutant receptors show different gating kinetics when activated by acetylcholine or by the noncompetitive agonist 5-hydroxytryptamine
Abstract
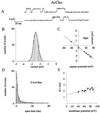
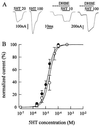
Mutation of the highly conserved leucine residue (Leu-247) converts 5-hydroxytryptamine (5HT) from an antagonist into an agonist of neuronal homomeric alpha7 nicotinic acetylcholine receptor expressed in Xenopus oocytes. We show here that acetylcholine (AcCho) activates two classes of single channels with conductances of 44 pS and 58 pS, similar to those activated by 5HT. However, the mean open time of AcCho-gated ion channels (11 ms) is briefer than that of 5HT-gated ion channels (18 ms). Furthermore, whereas the open time of AcCho channels lengthens with hyperpolarization, that of 5HT channels is decreased. In voltage-clamped oocytes, the apparent affinity of the alpha7 mutant receptor for 5HT is not modified by the presence of dihydro-beta-erythroidine, which acts on the AcCho binding site in a competitive manner. This indicates a noncompetitive action of 5HT on nicotinic acetylcholine receptors. Considered together, our findings show that AcCho gates alpha7 mutant channels with similar conductance but with different kinetic profile than the channels gated by 5HT, suggesting that the two agonists act on different docking sites. These results will help to understand the crosstalk between cholinergic and serotonergic systems in the central nervous system.
Figures
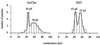

References
-
- McGehee D S, Role L. Curr Opin Neurobiol. 1996;6:342–349. - PubMed
-
- Wonnacot S. Trends Neurosci. 1997;20:92–98. - PubMed
-
- Zhang Z, Coggan J S, Berg D K. Neuron. 1996;17:1231–1240. - PubMed
-
- Clarke P, Quik M, Adikofer F, Thurau K, editors. Advances in Pharmacological Sciences. Vol. 2. Basel: Birkhauser; 1995.
-
- Maelicke A, Schrattenholz A, Schroder H. Semin Neurosci. 1995;7:103–114.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

