Cyclic AMP levels, adenylyl cyclase activity, and their stimulation by serotonin quantified in intact neurons
- PMID: 9276752
- PMCID: PMC2229365
- DOI: 10.1085/jgp.110.3.243
Cyclic AMP levels, adenylyl cyclase activity, and their stimulation by serotonin quantified in intact neurons
Abstract
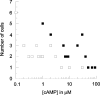
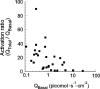
In molluscan central neurons that express cAMP-gated Na+ current (INa,cAMP), estimates of the cAMP binding affinity of the channels have suggested that effective native intracellular cAMP concentrations should be much higher than characteristic of most cells. Using neurons of the marine opisthobranch snail Pleurobranchaea californica, we applied theory and conventional voltage clamp techniques to use INa,cAMP to report basal levels of endogenous cAMP and adenylyl cyclase, and their stimulation by serotonin. Measurements were calibrated to iontophoretic cAMP injection currents to enable expression of the data in molar terms. In 30 neurons, serotonin stimulated on average a 23-fold increase in submembrane [cAMP], effected largely by an 18-fold increase in adenylyl cyclase activity. Serotonin stimulation of adenylyl cyclase and [cAMP] was inversely proportional to cells' resting adenylyl cyclase activity. Average cAMP concentration at the membrane rose from 3.6 to 27.6 microM, levels consistent with the expected cAMP dissociation constants of the INa,cAMP channels. These measures confirm the functional character of INa,cAMP in the context of high levels of native cAMP. Methods similar to those employed here might be used to establish critical characters of cyclic nucleotide metabolism in the many cells of invertebrates and vertebrates that are being found to express ion currents gated by direct binding of cyclic nucleotides.
Figures





References
-
- Aldenhoff JB, Hofmeier G, Lux HD, Swandulla D. Stimulation of a sodium influx by cAMP in Helixneurons. Brain Res. 1983;276:289–296. - PubMed
-
- Bacskai BJ, Hochner B, Mahaut-Smith M, Adams SR, Kaang B-K, Kandel ER, Tsien RY. Spatially resolved dynamics of cAMP and protein kinase A subunits in Aplysiasensory neurons. Science (Wash DC) 1993;260:222–226. - PubMed
-
- Bolger GB. Molecular biology of the cyclic AMP-specific cyclic nucleotide phosphodiesterases: a diverse family of regulatory enzymes. Cell Signal. 1994;6:851–859. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

