Allosteric gating of a large conductance Ca-activated K+ channel
- PMID: 9276753
- PMCID: PMC2229366
- DOI: 10.1085/jgp.110.3.257
Allosteric gating of a large conductance Ca-activated K+ channel
Abstract
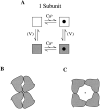
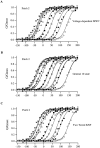
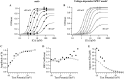
Large-conductance Ca-activated potassium channels (BK channels) are uniquely sensitive to both membrane potential and intracellular Ca2+. Recent work has demonstrated that in the gating of these channels there are voltage-sensitive steps that are separate from Ca2+ binding steps. Based on this result and the macroscopic steady state and kinetic properties of the cloned BK channel mslo, we have recently proposed a general kinetic scheme to describe the interaction between voltage and Ca2+ in the gating of the mslo channel (Cui, J., D.H. Cox, and R.W. Aldrich. 1997. J. Gen. Physiol. In press.). This scheme supposes that the channel exists in two main conformations, closed and open. The conformational change between closed and open is voltage dependent. Ca2+ binds to both the closed and open conformations, but on average binds more tightly to the open conformation and thereby promotes channel opening. Here we describe the basic properties of models of this form and test their ability to mimic mslo macroscopic steady state and kinetic behavior. The simplest form of this scheme corresponds to a voltage-dependent version of the Monod-Wyman-Changeux (MWC) model of allosteric proteins. The success of voltage-dependent MWC models in describing many aspects of mslo gating suggests that these channels may share a common molecular mechanism with other allosteric proteins whose behaviors have been modeled using the MWC formalism. We also demonstrate how this scheme can arise as a simplification of a more complex scheme that is based on the premise that the channel is a homotetramer with a single Ca2+ binding site and a single voltage sensor in each subunit. Aspects of the mslo data not well fitted by the simplified scheme will likely be better accounted for by this more general scheme. The kinetic schemes discussed in this paper may be useful in interpreting the effects of BK channel modifications or mutations.
Figures












References
-
- Adelman JP, Shen KZ, Kavanaugh MP, Warren RA, Wu YN, Lagrutta A, Bond CT, North RA. Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron. 1992;9:209–216. - PubMed
-
- Aggarwal SK, MacKinnon R. Contribution of the S4 segment to gating charge in the shaker K+channel. Neuron. 1996;16:1169–1177. - PubMed
-
- Atkinson NS, Robertson GA, Ganetzky B. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science (Wash DC) 1991;253:551–555. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

