Trk receptors function as rapid retrograde signal carriers in the adult nervous system
- PMID: 9278536
- PMCID: PMC6573276
- DOI: 10.1523/JNEUROSCI.17-18-07007.1997
Trk receptors function as rapid retrograde signal carriers in the adult nervous system
Abstract
During development target-derived neurotrophins promote the survival of neurons. However, mature neurons no longer depend on the target for survival. Do target-derived neurotrophins retain retrograde signaling functions in mature neurons, and, if so, how are they executed? We addressed this question by using a phosphotyrosine-directed antibody to locate activated Trk receptors in adult rat sciatic nerve. We show that catalytically active Trk receptors are located within the axon of adult rat sciatic nerve and that they are distributed throughout the length of the axons. These catalytically active receptors are phosphorylated on tyrosine at a position that couples them to the signal-generating proteins Ras and PI3 kinase. Neurotrophin applied at sciatic nerve terminals increases both catalytic activity and phosphorylation state of Trk receptors at distant points within the axons. Trk activation initiated at the nerve terminals propagates through the axon toward the nerve cell body at an initial rate that exceeds that of conventional vesicular transport. However, our data suggest that this rapid signal is nevertheless vesicle-associated. Thus, in mature nerves, activated Trk receptors function as rapid retrograde signal carriers to execute remote responses to target-derived neurotrophins.
Figures

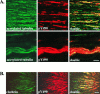
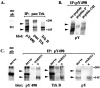

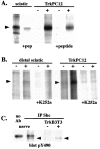
References
-
- Acheson A, Conover J, Fandl J, DeChiara T, Russell M, Thadani A, Squinto S, Yancopoulos G, Lindsay R. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature. 1995;374:450–453. - PubMed
-
- Barbacid M. The Trk family of neurotrophin receptors. J Neurobiol. 1994;25:1386–1403. - PubMed
-
- Barde YA. Trophic factors and neuronal survival. Neuron. 1989;2:1525–1534. - PubMed
-
- Bothwell M. Keeping track of neurotrophin receptors. Cell. 1991;65:915–918. - PubMed
-
- Campenot RB. Development of sympathetic neurons in compartmentalized cultures. I. Local control of neurite growth by nerve growth factor. Dev Biol. 1982;93:1–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
