Integrin alpha2beta1 is a receptor for the cartilage matrix protein chondroadherin
- PMID: 9281592
- PMCID: PMC2136766
- DOI: 10.1083/jcb.138.5.1159
Integrin alpha2beta1 is a receptor for the cartilage matrix protein chondroadherin
Abstract
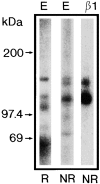
Chondroadherin (the 36-kD protein) is a leucine-rich, cartilage matrix protein known to mediate adhesion of isolated chondrocytes. In the present study we investigated cell surface proteins involved in the interaction of cells with chondroadherin in cell adhesion and by affinity purification. Adhesion of bovine articular chondrocytes to chondroadherin-coated dishes was dependent on Mg2+ or Mn2+ but not Ca2+. Adhesion was partially inhibited by an antibody recognizing beta1 integrin subunit. Chondroadherin-binding proteins from chondrocyte lysates were affinity purified on chondroadherin-Sepharose. The beta1 integrin antibody immunoprecipitated two proteins with molecular mass approximately 110 and 140 kD (nonreduced) from the EDTA-eluted material. These results indicate that a beta1 integrin on chondrocytes interacts with chondroadherin. To identify the alpha integrin subunit(s) involved in interaction of cells with the protein, we affinity purified chondroadherin-binding membrane proteins from human fibroblasts. Immunoprecipitation of the EDTA-eluted material from the affinity column identified alpha2beta1 as a chondroadherin-binding integrin. These results are in agreement with cell adhesion experiments where antibodies against the integrin subunit alpha2 partially inhibited adhesion of human fibroblast and human chondrocytes to chondroadherin. Since alpha2beta1 also is a receptor for collagen type II, we tested the ability of different antibodies against the alpha2 subunit to inhibit adhesion of T47D cells to collagen type II and chondroadherin. The results suggested that adhesion to collagen type II and chondroadherin involves similar or nearby sites on the alpha2beta1 integrin. Although alpha2beta1 is a receptor for both collagen type II and chondroadherin, only adhesion of cells to collagen type II was found to mediate spreading.
Figures






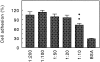



References
-
- Bengtsson E, Neames PJ, Heinegard D, Sommarin Y. The primary structure of a basic leucine-rich repeat protein, PRELP, found in connective tissue. J Biol Chem. 1995;270:25639–25644. - PubMed
-
- Blochberger TC, Vergnes J-P, Hempel J, Hassell JR. cDNA to chick lumican (corneal keratan sulfate proteoglycan) reveals homology to the small interstitial proteoglycan gene family and expression in muscle and intestine. J Biol Chem. 1992;267:347–352. - PubMed
-
- Chen JD, Kim JP, Zhang K, Sarret Y, Wynn KC, Kramer RH, Woodley DT. Epidermal growth factor (EGF) promotes human keratinocyte locomotion on collagen by increasing the α2 integrin subunit. Exp Cell Res. 1993;209:216–223. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

