Persistent activation by and receptor reserve for an irreversible A1-adenosine receptor agonist in DDT1 MF-2 cells and in guinea pig heart
- PMID: 9281612
- PMCID: PMC5472056
- DOI: 10.1124/mol.52.3.491
Persistent activation by and receptor reserve for an irreversible A1-adenosine receptor agonist in DDT1 MF-2 cells and in guinea pig heart
Abstract
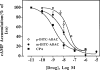
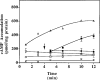
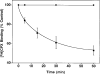
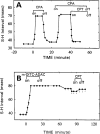
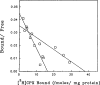
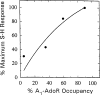
The p- and m-isothiocyanate adenosine derivatives N6-[4-[[[4-[[[[2-[[[(p-(m)-isothiocyanatophenyl)amino]thiocarbonyl ]am ino]ethyl]amino]carbonyl]methyl]anilino]carbonyl]methyl]phenyl] adenosine (p- and m-DITC-ADAC) were examined for irreversible agonist effects at the A1-adenosine receptor (A1-AdoR) in DDT1 MF-2 (DDT) cells and a functional A1-AdoR response in the guinea pig isolated heart. The p- and m-DITC-ADAC inhibited (-)-isoproterenol stimulated cAMP accumulation in DDT cells in the low nanomolar range, and the maximal responses elicited by both compounds were similar to that for N6-cyclopentyladenosine. Once established, the p-DITC-ADAC-mediated inhibition of cAMP accumulation in DDT cells was not affected by the addition of the AdoR antagonist 8-cyclopentyl-1,3-dipropylxanthine (CPX). Pretreatment of DDT cells with p-DITC-ADAC (1 microM), followed by washing, reduced [3H]CPX binding to the A1-AdoR by 44% without altering the Kd value for the radioligand to the remaining receptors. The relationship between irreversible A1-AdoR occupancy by p-DITC-ADAC and inhibition of cAMP accumulation revealed a relatively large receptor reserve (64%) for the maximal response. In guinea pig isolated hearts, m-DITC-ADAC (5 microM) prolonged the stimulus to His bundle (SH) interval by 2.1-fold; this response could be prevented by the antagonist 8-cyclopentyltheophylline (5 microM). However, after the SH interval prolongation was established, extensive washout or the addition of 8-cyclopentyltheophylline had little reversal effect on the m-DITC-ADAC response. Binding of [3H]CPX to the guinea pig ventricular membranes after m-DITC-ADAC treatment and washing was reduced by 35%. The A1-AdoR occupancy response relationship for m-DITC-ADAC to prolong the SH interval indicated a small (10-20%) receptor reserve. Both p -and m-DITC-ADAC seem to be irreversible full agonists at the A1-AdoR and may prove to be useful probes to further investigate A1-AdoR structure-function relationships.
Figures




Similar articles
-
Evidence of spare A1-adenosine receptors in guinea pig atrioventricular node.Am J Physiol. 1992 Mar;262(3 Pt 2):H661-71. doi: 10.1152/ajpheart.1992.262.3.H661. Am J Physiol. 1992. PMID: 1558173 Free PMC article.
-
1,3-Dipropyl-8-[2-(5,6-epoxy)norbornyl]xanthine, a potent, specific and selective A1 adenosine receptor antagonist in the guinea pig heart and brain and in DDT1MF-2 cells.J Pharmacol Exp Ther. 1995 Dec;275(3):1167-76. J Pharmacol Exp Ther. 1995. PMID: 8531078
-
A novel irreversible antagonist of the A1-adenosine receptor.Mol Pharmacol. 1996 Jul;50(1):196-205. Mol Pharmacol. 1996. PMID: 8700113
-
A1 adenosine receptor inhibition of cyclic AMP formation and radioligand binding in the guinea-pig cerebral cortex.Br J Pharmacol. 1994 Dec;113(4):1501-7. doi: 10.1111/j.1476-5381.1994.tb17166.x. Br J Pharmacol. 1994. PMID: 7889308 Free PMC article.
-
A novel partial agonist of the A(1)-adenosine receptor and evidence of receptor homogeneity in adipocytes.J Pharmacol Exp Ther. 2006 May;317(2):676-84. doi: 10.1124/jpet.105.099119. Epub 2006 Jan 12. J Pharmacol Exp Ther. 2006. PMID: 16410404
Cited by
-
Development of subtype-selective covalent ligands for the adenosine A2B receptor by tuning the reactive group.RSC Med Chem. 2022 Jun 21;13(7):850-856. doi: 10.1039/d2md00132b. eCollection 2022 Jul 20. RSC Med Chem. 2022. PMID: 35923720 Free PMC article.
-
Adenosine A1 and A3 Receptors: Distinct Cardioprotection.Drug Dev Res. 2001 Jan-Feb;52(1-2):366-378. doi: 10.1002/ddr.1136. Drug Dev Res. 2001. PMID: 39741902 Free PMC article.
-
Adenosine Receptor Reserve and Long-Term Potentiation: Unconventional Adaptive Mechanisms in Cardiovascular Diseases?Int J Mol Sci. 2021 Jul 15;22(14):7584. doi: 10.3390/ijms22147584. Int J Mol Sci. 2021. PMID: 34299203 Free PMC article. Review.
-
Functionalized congeners of 1,4-dihydropyridines as antagonist molecular probes for A3 adenosine receptors.Bioconjug Chem. 1999 Jul-Aug;10(4):667-77. doi: 10.1021/bc9900136. Bioconjug Chem. 1999. PMID: 10411465 Free PMC article.
-
Chemical Probes for the Adenosine Receptors.Pharmaceuticals (Basel). 2019 Nov 12;12(4):168. doi: 10.3390/ph12040168. Pharmaceuticals (Basel). 2019. PMID: 31726680 Free PMC article. Review.
References
-
- Collis MG, Hourani SMO. Adenosine receptor subtypes. Trends Pharmacol Sci. 1993;14:360–366. - PubMed
-
- Linden J. Cloned adenosine A3 receptors: pharmacological properties, species differences and receptor functions. Trends Pharmacol Sci. 1994;15:298–306. - PubMed
-
- Belardinelli L, Shryock JC, Wang SD, Srinivas M. Ionic basis of the electrophysiological actions of adenosine on cardiomyocytes. FASEB J. 1995;9:369–365. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

