Drosophila P-element transposase is a novel site-specific endonuclease
- PMID: 9284052
- PMCID: PMC316450
- DOI: 10.1101/gad.11.16.2137
Drosophila P-element transposase is a novel site-specific endonuclease
Abstract
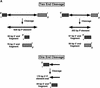
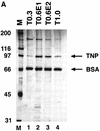
We developed in vitro assays to study the first step of the P-element transposition reaction: donor DNA cleavage. We found that P-element transposase required both 5' and 3' P-element termini for efficient DNA cleavage to occur, suggesting that a synaptic complex forms prior to cleavage. Transposase made a staggered cleavage at the P-element termini that is novel for all known site-specific endonucleases: the 3' cleavage site is at the end of the P-element, whereas the 5' cleavage site is 17 bp within the P-element 31-bp inverted repeats. The P-element termini were protected from exonucleolytic degradation following the cleavage reaction, suggesting that a stable protein complex remains bound to the element termini after cleavage. These data are consistent with a cut-and-paste mechanism for P-element transposition and may explain why P elements predominantly excise imprecisely in vivo.
Figures












References
-
- Agrawal A, Schatz DG. RAG1 and RAG2 form a stable postcleavage synaptic complex with DNA containing signal ends in V(D)J recombination. Cell. 1997;89:43–53. - PubMed
-
- Anderson CW, Lees-Miller SP. The nuclear serine/threonine protein kinase DNA-PK. Crit Rev Eukaryot Gene Expression. 1992;2:283–314. - PubMed
-
- Bainton R, Gamas P, Craig NL. Tn7 transposition in vitro proceeds through an excised transposon intermediate generated by staggered breaks in DNA. Cell. 1991;65:805–816. - PubMed
-
- Bainton RJ, Kubo KM, Feng JN, Craig NL. Tn7 transposition: Target DNA recognition is mediated by multiple Tn7-encoded proteins in a purified in vitro system. Cell. 1993;72:931–943. - PubMed
-
- Beall EL, Rio DC. Drosophila IRBP/Ku p70 corresponds to the mutagen-sensitive mus309 gene and is involved in P-element excision in vivo. Genes & Dev. 1996;10:921–933. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
