ActA is a dimer
- PMID: 9294158
- PMCID: PMC23296
- DOI: 10.1073/pnas.94.19.10034
ActA is a dimer
Abstract
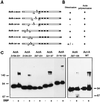
ActA, a surface protein of Listeria monocytogenes, is able to induce continuous actin polymerization at the rear of the bacterium, in the cytosol of the infected cells. Its N-terminal domain is sufficient to induce actin tail formation and movement. Here, we demonstrate, using the yeast two-hybrid system, that the N-terminal domain of ActA may form homodimers. By using chemical cross-linking to explore the possibility that ActA could be a multimer on the surface of the bacteria, we show that ActA is a dimer. Cross-linking experiments on various L. monocytogenes strains expressing different ActA variants demonstrated that the region spanning amino acids 97-126, and previously identified as critical for actin tail formation, is also critical for dimer formation. A model of actin polymerization by L. monocytogenes, involving the ActA dimer, is presented.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

