The activation domain of the enhancer binding protein p45NF-E2 interacts with TAFII130 and mediates long-range activation of the alpha- and beta-globin gene loci in an erythroid cell line
- PMID: 9294161
- PMCID: PMC23301
- DOI: 10.1073/pnas.94.19.10051
The activation domain of the enhancer binding protein p45NF-E2 interacts with TAFII130 and mediates long-range activation of the alpha- and beta-globin gene loci in an erythroid cell line
Abstract
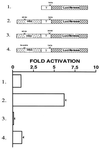
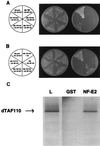
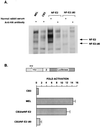
We have used the interaction between the erythroid-specific enhancer in hypersensitivity site 2 of the human beta-globin locus control region and the globin gene promoters as a paradigm to examine the mechanisms governing promoter/enhancer interactions in this locus. We have demonstrated that enhancer-dependent activation of the globin promoters is dependent on the presence of both a TATA box in the proximal promoter and the binding site for the erythroid-specific heteromeric transcription factor NF-E2 in the enhancer. Mutational analysis of the transcriptionally active component of NF-E2, p45NF-E2, localizes the critical region for this function to a proline-rich transcriptional activation domain in the NH2-terminal 80 amino acids of the protein. In contrast to the wild-type protein, expression of p45 NF-E2 lacking this activation domain in an NF-E2 null cell line fails to support enhancer-dependent transcription in transient assays. More significantly, the mutated protein also fails to reactivate expression of the endogenous beta- or alpha-globin loci in this cell line. Protein-protein interaction studies reveal that this domain of p45 NF-E2 binds specifically to a component of the transcription initiation complex, TATA binding protein associated factor TAFII130. These findings suggest one potential mechanism for direct recruitment of distal regulatory regions of the globin loci to the individual promoters.
Figures


References
-
- Tjian R, Maniatis T. Cell. 1994;77:5–8. - PubMed
-
- McKnight S L. Genes Dev. 1996;10:367–381. - PubMed
-
- Tanese N, Tjian R. Cold Spring Harb Symp Quant Biol. 1993;58:179–185. - PubMed
-
- Sauer F, Hansen S K, Tjian R. Science. 1995;270:1783–1788. - PubMed
-
- Chiang C M, Roeder R G. Science. 1995;267:531–536. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

