Probing the role of packing specificity in protein design
- PMID: 9294182
- PMCID: PMC23334
- DOI: 10.1073/pnas.94.19.10172
Probing the role of packing specificity in protein design
Abstract
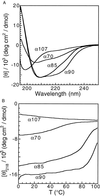
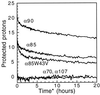
By using a protein-design algorithm that quantitatively considers side-chain packing, the effect of specific steric constraints on protein design was assessed in the core of the streptococcal protein G beta1 domain. The strength of packing constraints used in the design was varied, resulting in core sequences that reflected differing amounts of packing specificity. The structural flexibility and stability of several of the designed proteins were experimentally determined and showed a trend from well-ordered to highly mobile structures as the degree of packing specificity in the design decreased. This trend both demonstrates that the inclusion of specific packing interactions is necessary for the design of native-like proteins and defines a useful range of packing specificity for the design algorithm. In addition, an analysis of the modeled protein structures suggested that penalizing for exposed hydrophobic surface area can improve design performance.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

