Beta-catenin mutations in cell lines established from human colorectal cancers
- PMID: 9294210
- PMCID: PMC23362
- DOI: 10.1073/pnas.94.19.10330
Beta-catenin mutations in cell lines established from human colorectal cancers
Abstract
beta-catenin has functions as both an adhesion and a signaling molecule. Disruption of these functions through mutations of the beta-catenin gene (CTNNB1) may be important in the development of colorectal tumors. We examined the entire coding sequence of beta-catenin by reverse transcriptase-PCR (RT-PCR) and direct sequencing of 23 human colorectal cancer cell lines from 21 patients. In two cell lines, there was apparent instability of the beta-catenin mRNA. Five different mutations (26%) were found in the remaining 21cell lines (from 19 patients). A three-base deletion (codon 45) was identified in the cell line HCT 116, whereas cell lines SW 48, HCA 46, CACO 2, and Colo 201 each contained single-base missense mutations (codons 33, 183, 245, and 287, respectively). All 23 cell lines had full-length beta-catenin protein that was detectable by Western blotting and that coprecipitated with E-cadherin. In three of the cell lines with CTNNB1 mutations, complexes of beta-catenin with alpha-catenin and APC were detectable. In SW48 and HCA 46, however, we did not detect complexes of beta-catenin protein with alpha-catenin and APC, respectively. These results show that selection of CTNNB1 mutations occurs in up to 26% of colorectal cancers from which cell lines are derived. In these cases, mutation selection is probably for altered beta-catenin function, which may significantly alter intracellular signaling and intercellular adhesion and may serve as a complement to APC mutations in the early stages of tumorigenesis.
Figures
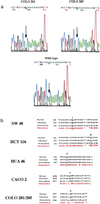
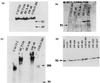
References
-
- Peifer M, Berg S, Reynolds A B. Cell. 1994;76:789–791. - PubMed
-
- Peifer M. Trends Cell Biol. 1995;5:224–229. - PubMed
-
- McCrea P D, Turck C W, Gumbiner B. Science. 1991;254:1359–1361. - PubMed
-
- Gumbiner B M, McCrea P D. J Cell Sci Suppl. 1993;17:155–158. - PubMed
-
- Gumbiner B M. Curr Opin Cell Biol. 1995;7:634–640. - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

