Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death
- PMID: 9294220
- PMCID: PMC23372
- DOI: 10.1073/pnas.94.19.10385
Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death
Abstract
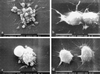
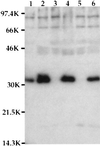
Pathogenic Yersinia spp. carry a large common plasmid that encodes a number of essential virulence determinants. Included in these factors are the Yersinia-secreted proteins called Yops. We analyzed the consequences of wild-type and mutant strains of Yersinia pseudotuberculosis interactions with the macrophage cell line RAW264. 7 and murine bone marrow-derived macrophages. Wild-type Y. pseudotuberculosis kills approximately 70% of infected RAW264.7 macrophages and marrow-derived macrophages after an 8-h infection. We show that the cell death mediated by Y. pseudotuberculosis is apoptosis. Mutant Y. pseudotuberculosis that do not make any Yop proteins no longer cause host cell death. Attachment to host cells via invasin or YadA is necessary for the cell death phenotype. Several Yop mutant strains that fail to express one or more Yop proteins were engineered and then characterized for their ability to cause host cell death. A mutant with a polar insertion in YpkA Ser/Thr kinase that does not express YpkA or YopJ is no longer able to cause apoptosis. In contrast, a mutant no longer making YopE or YopH (a tyrosine phosphatase) induces apoptosis in macrophages similar to wild type. When yopJ is added in trans to the ypkAyopJ mutant, the ability of this strain to signal programmed cell death in macrophages is restored. Thus, YopJ is necessary for inducing apoptosis. The ability of Y. pseudotuberculosis to promote apoptosis of macrophages in cell culture suggests that this process is important for the establishment of infection in the host and for evasion of the host immune response.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

