Neuroprotective activity of a new class of steroidal inhibitors of the N-methyl-D-aspartate receptor
- PMID: 9294231
- PMCID: PMC23383
- DOI: 10.1073/pnas.94.19.10450
Neuroprotective activity of a new class of steroidal inhibitors of the N-methyl-D-aspartate receptor
Abstract
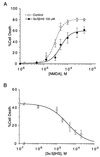
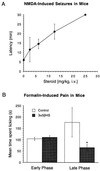
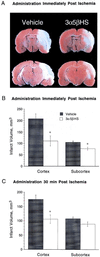
Release of the excitatory neurotransmitter glutamate and the excessive stimulation of N-methyl-D-aspartate (NMDA)-type glutamate receptors is thought to be responsible for much of the neuronal death that occurs following focal hypoxia-ischemia in the central nervous system. Our laboratory has identified endogenous sulfated steroids that potentiate or inhibit NMDA-induced currents. Here we report that 3alpha-ol-5beta-pregnan-20-one hemisuccinate (3alpha5betaHS), a synthetic homologue of naturally occurring pregnanolone sulfate, inhibits NMDA-induced currents and cell death in primary cultures of rat hippocampal neurons. 3alpha5betaHS exhibits sedative, anticonvulsant, and analgesic properties consistent with an action at NMDA-type glutamate receptors. Intravenous administration of 3alpha5betaHS to rats (at a nonsedating dose) following focal cerebral ischemia induced by middle cerebral artery occlusion significantly reduces cortical and subcortical infarct size. The in vitro and in vivo neuroprotective effects of 3alpha5betaHS demonstrate that this steroid represents a new class of potentially useful therapeutic agents for the treatment of stroke and certain neurodegenerative diseases that involve over activation of NMDA receptors.
Figures


References
-
- MacDermott A, Mayer M, Westbrook G, Smith S, Barker S. Nature (London) 1986;321:519–522. - PubMed
-
- Choi D. Neurosci Lett. 1985;58:293–297. - PubMed
-
- Puia G, Santi M, Vicini S, Pritchett D B, Purdy R H, Paul S M, Seeburg P H, Costa E. Neuron. 1990;4:759–765. - PubMed
-
- Farb D H, Gibbs T T, Wu F-S, Gyenes M, Friedman L, Russek S J. In: Steroid Modulation of Amino Acid Neurotransmitter Receptors. Biggio G, Concas A, Costa E, editors. Vol. 47. New York: Raven; 1992. pp. 119–131. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

