RGS proteins reconstitute the rapid gating kinetics of gbetagamma-activated inwardly rectifying K+ channels
- PMID: 9294233
- PMCID: PMC23385
- DOI: 10.1073/pnas.94.19.10461
RGS proteins reconstitute the rapid gating kinetics of gbetagamma-activated inwardly rectifying K+ channels
Abstract
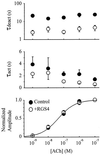
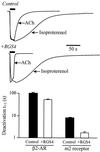
G protein-gated inward rectifier K+ (GIRK) channels mediate hyperpolarizing postsynaptic potentials in the nervous system and in the heart during activation of Galpha(i/o)-coupled receptors. In neurons and cardiac atrial cells the time course for receptor-mediated GIRK current deactivation is 20-40 times faster than that observed in heterologous systems expressing cloned receptors and GIRK channels, suggesting that an additional component(s) is required to confer the rapid kinetic properties of the native transduction pathway. We report here that heterologous expression of "regulators of G protein signaling" (RGS proteins), along with cloned G protein-coupled receptors and GIRK channels, reconstitutes the temporal properties of the native receptor --> GIRK signal transduction pathway. GIRK current waveforms evoked by agonist activation of muscarinic m2 receptors or serotonin 1A receptors were dramatically accelerated by coexpression of either RGS1, RGS3, or RGS4, but not RGS2. For the brain-expressed RGS4 isoform, neither the current amplitude nor the steady-state agonist dose-response relationship was significantly affected by RGS expression, although the agonist-independent "basal" GIRK current was suppressed by approximately 40%. Because GIRK activation and deactivation kinetics are the limiting rates for the onset and termination of "slow" postsynaptic inhibitory currents in neurons and atrial cells, RGS proteins may play crucial roles in the timing of information transfer within the brain and to peripheral tissues.
Figures
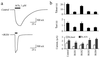


References
-
- Druey K M, Blumer K J, Kang V H, Kehrl J H. Nature (London) 1996;379:742–746. - PubMed
-
- Koelle M R, Horvitz H R. Cell. 1996;84:115–125. - PubMed
-
- Berman D M, Wilkie T M, Gilman A G. Cell. 1996;86:445–452. - PubMed
-
- Watson N, Linder M E, Druey K M, Kehrl J H, Blumer K J. Nature (London) 1996;383:172–177. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

