Rapid synaptic transmission in the avian ciliary ganglion is mediated by two distinct classes of nicotinic receptors
- PMID: 9295367
- PMCID: PMC6573447
- DOI: 10.1523/JNEUROSCI.17-19-07210.1997
Rapid synaptic transmission in the avian ciliary ganglion is mediated by two distinct classes of nicotinic receptors
Abstract
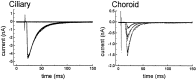
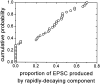
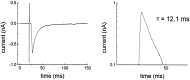
We analyzed the kinetics and pharmacology of EPSCs in two kinds of neurons in the embryonic avian ciliary ganglion. Whole-cell voltage-clamp recordings revealed that the singly innervated ciliary neurons had large-amplitude (1.5-8.0 nA) EPSCs that could be classified according to the kinetics of their falling phases. Most of the neurons responded with an EPSC the falling phase of which followed a double exponential time course with time constants of approximately 1 and 10 msec. The EPSCs of the remaining ciliary neurons followed a single time constant ( approximately 8 msec). Multiple innervated choroid neurons had smaller-amplitude responses (0.2-1.5 nA when all inputs were activated) that appeared to contain only a slowly decaying component (tau = 12 msec). The fast and slow components of EPSC decay seen in most ciliary neurons could be pharmacologically isolated with two toxins against nicotinic acetylcholine receptors (AChRs). The fast component was blocked by 50 nM alpha-bungarotoxin (alpha-BuTx), which binds alpha7-subunit-containing AChRs. The slow component was selectively blocked by 50 nM alpha-conotoxin MII (alpha-CTx-MII), which blocks mammalian AChRs containing an alpha3/beta2 subunit interface. A combination of both alpha-BuTx and alpha-CTx-MII abolished nearly all evoked current. Similar pharmacological results were found for ciliary neurons with monoexponentially decaying EPSCs and for choroid neurons. These results suggest that nerve-evoked transmitter acts on at least two different populations of AChRs on autonomic motor neurons in the ciliary ganglion.
Figures






References
-
- Albuquerque EX, Alkondon M, Pereira EF, Castro NG, Schrattenholz A, Barbosa CT, Bonfante-Cabarcas R, Aracava Y, Eisenberg HM, Maelicke A. Properties of neuronal nicotinic acetylcholine receptors: pharmacological characterization and modulation of synaptic function. J Pharmacol Exp Ther. 1997;280:1117–1136. - PubMed
-
- Anand R, Peng X, Lindstrom J. Homomeric and native alpha7 acetylcholine receptors exhibit remarkably similar but non-identical pharmacological properties, suggesting that the native receptor is a heteromeric protein complex. FEBS Lett. 1993;327:241–246. - PubMed
-
- Boyd RT, Jacob MH, Couturier S, Ballivet M, Berg DK. Expression and regulation of neuronal acetylcholine receptor mRNA in chick ciliary ganglia. Neuron. 1988;1:495–502. - PubMed
-
- Cartier GE, Yoshikami DJ, Gray WR, Luo SQ, Olivera BM, McIntosh JM. A new alpha-conotoxin which targets alpha-3-beta-2 nicotinic acetylcholine receptors. J Biol Chem. 1996;271:7522–7528. - PubMed
-
- Chiappinelli VA, Dryer SE. Nicotinic transmission in sympathetic ganglia: blockade by the snake venom neurotoxin kappa-bungarotoxin. Neurosci Lett. 1984;50:239–244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
