Impact of cytoplasmic calcium buffering on the spatial and temporal characteristics of intercellular calcium signals in astrocytes
- PMID: 9295382
- PMCID: PMC6573438
- DOI: 10.1523/JNEUROSCI.17-19-07359.1997
Impact of cytoplasmic calcium buffering on the spatial and temporal characteristics of intercellular calcium signals in astrocytes
Abstract
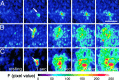
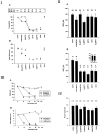
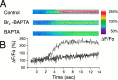
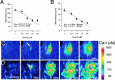
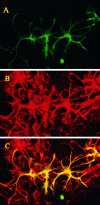
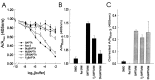
The impact of calcium buffering on the initiation and propagation of mechanically elicited intercellular Ca2+ waves was studied using astrocytes loaded with different exogenous, cell membrane-permeant Ca2+ chelators and a laser scanning confocal or video fluorescence microscope. Using an ELISA with a novel antibody to BAPTA, we showed that different cell-permeant chelators, when applied at the same concentrations, accumulate to the same degree inside the cells. Loading cultures with BAPTA, a high Ca2+ affinity chelator, almost completely blocked calcium wave occurrence. Chelators having lower Ca2+ affinities had lesser affects, as shown in their attenuation of both the radius of spread and propagation velocity of the Ca2+ wave. The chelators blocked the process of wave propagation, not initiation, because large [Ca2+]i increases elicited in the mechanically stimulated cell were insufficient to trigger the wave in the presence of high Ca2+ affinity buffers. Wave attenuation was a function of cytoplasmic Ca2+ buffering capacity; i.e., loading increasing concentrations of low Ca2+ affinity buffers mimicked the effects of lesser quantities of high-affinity chelators. In chelator-treated astrocytes, changes in calcium wave properties were independent of the Ca2+-binding rate constants of the chelators, of chelation of other ions such as Zn2+, and of effects on gap junction function. Slowing of the wave could be completely accounted for by the slowing of Ca2+ ion diffusion within the cytoplasm of individual astrocytes. The data obtained suggest that alterations in Ca2+ buffering may provide a potent mechanism by which the localized spread of astrocytic Ca2+ signals is controlled.
Figures
References
-
- Allbritton NL, Meyer T, Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-triphosphate. Science. 1992;258:1812–1815. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
