Neural coding mechanisms in tactile pattern recognition: the relative contributions of slowly and rapidly adapting mechanoreceptors to perceived roughness
- PMID: 9295394
- PMCID: PMC6573449
- DOI: 10.1523/JNEUROSCI.17-19-07480.1997
Neural coding mechanisms in tactile pattern recognition: the relative contributions of slowly and rapidly adapting mechanoreceptors to perceived roughness
Abstract
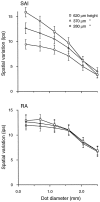
Tactile pattern recognition depends on form and texture perception. A principal dimension of texture perception is roughness, the neural coding of which was the focus of this study. Previous studies have shown that perceived roughness is not based on neural activity in the Pacinian or cutaneous slowly adapting type II (SAII) neural responses or on mean impulse rate or temporal patterning in the cutaneous slowly adapting type I (SAI) or rapidly adapting (RA) discharge evoked by a textured surface. However, those studies found very high correlations between roughness scaling by humans and measures of spatial variation in SAI and RA firing rates. The present study used textured surfaces composed of dots of varying height (280-620 micron) and diameter (0.25-2.5 mm) in psychophysical and neurophysiological experiments. RA responses were affected least by the range of dot diameters and heights that produced the widest variation in perceived roughness, and these responses could not account for the psychophysical data. In contrast, spatial variation in SAI impulse rate was correlated closely with perceived roughness over the whole stimulus range, and a single measure of SAI spatial variation accounts for the psychophysical data in this (0.974 correlation) and two previous studies. Analyses based on the possibility that perceived roughness depends on both afferent types suggest that if the RA response plays a role in roughness perception, it is one of mild inhibition. These data reinforce the hypothesis that SAI afferents are mainly responsible for information about form and texture whereas RA afferents are mainly responsible for information about flutter, slip, and motion across the skin surface.
Figures







References
-
- Blake DT, Hsiao SS, Johnson KO (1997) Slowly and rapidly adapting mechanoreceptive responses to raised and depressed scanned patterns: effects of width, height, orientation, and a raised surround. J Neurophysiol, in press. - PubMed
-
- Burton H, Sinclair RJ. Representation of tactile roughness in thalamus and somatosensory cortex. Can J Physiol Pharmacol. 1994;72:546–557. - PubMed
-
- Johansson RS, Westling G. Signals in tactile afferents from the fingers eliciting adaptive motor responses during precision grip. Exp Brain Res. 1987;66:141–154. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
