Transportin-mediated nuclear import of heterogeneous nuclear RNP proteins
- PMID: 9298975
- PMCID: PMC2132560
- DOI: 10.1083/jcb.138.6.1181
Transportin-mediated nuclear import of heterogeneous nuclear RNP proteins
Abstract
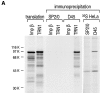
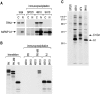
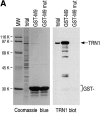
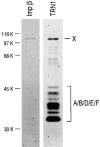
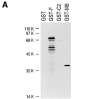
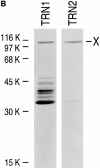
Heterogeneous nuclear ribonucleoprotein (hnRNP) A1 is an abundant nuclear protein that plays an important role in pre-mRNA processing and mRNA export from the nucleus. A1 shuttles rapidly between the nucleus and the cytoplasm, and a 38-amino acid domain, M9, serves as the bidirectional transport signal of A1. Recently, a 90-kD protein, transportin, was identified as the mediator of A1 nuclear import. In this study, we show that transportin mediates the nuclear import of additional hnRNP proteins, including hnRNP F. We have also isolated and sequenced a novel transportin homolog, transportin2, which may differ from transportin1 in its substrate specificity. Immunostaining shows that transportin1 is localized both in the cytoplasm and the nucleoplasm, and nuclear rim staining is also observed. The nuclear localization of A1 is dependent on ongoing RNA polymerase II transcription. Interestingly, a pyruvate kinase-M9 fusion, which normally localizes in the nucleus, also accumulates in the cytoplasm when RNA polymerase II is inhibited. Thus, M9 itself is a specific sensor for transcription-dependent nuclear transport. Transportin1-A1 complexes can be isolated from the cytoplasm and the nucleoplasm, but transportin1 is not detectable in hnRNP complexes. RanGTP causes dissociation of A1-transportin1 complexes in vitro. Thus, it is likely that after nuclear import, A1 dissociates from transportin1 by RanGTP and becomes incorporated into hnRNP complexes, where A1 functions in pre-mRNA processing.
Figures







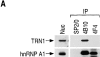
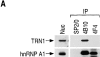

References
-
- Adam SA, Gerace L. Cytosolic proteins that specifically bind nuclear localization signals are receptors for nuclear import. Cell. 1991;66:837–847. - PubMed
-
- Aitchison JD, Blobel G, Rout MP. Kap104p: a karyopherin involved in the nuclear transport of messenger RNA binding proteins. Science (Wash DC) 1996;274:624–627. - PubMed
-
- Becker J, Melchior F, Gerke V, Bischoff FR, Ponstingl H, Wittinghofer A. RNA1 encodes a GTPase-activating protein specific for Gsp1p, the Ran/TC4 homologue of Saccharomyces cerevisiae. . J Biol Chem. 1995;270:11860–11865. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

