Nuclear membrane dynamics and reassembly in living cells: targeting of an inner nuclear membrane protein in interphase and mitosis
- PMID: 9298976
- PMCID: PMC2132565
- DOI: 10.1083/jcb.138.6.1193
Nuclear membrane dynamics and reassembly in living cells: targeting of an inner nuclear membrane protein in interphase and mitosis
Abstract
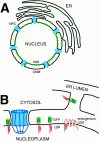
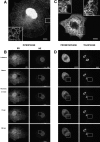
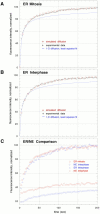
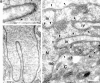
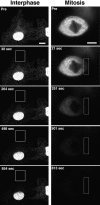
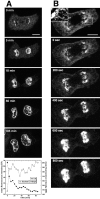
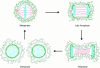
The mechanisms of localization and retention of membrane proteins in the inner nuclear membrane and the fate of this membrane system during mitosis were studied in living cells using the inner nuclear membrane protein, lamin B receptor, fused to green fluorescent protein (LBR-GFP). Photobleaching techniques revealed the majority of LBR-GFP to be completely immobilized in the nuclear envelope (NE) of interphase cells, suggesting a tight binding to heterochromatin and/or lamins. A subpopulation of LBR-GFP within ER membranes, by contrast, was entirely mobile and diffused rapidly and freely (D = 0. 41 +/- 0.1 microm2/s). High resolution confocal time-lapse imaging in mitotic cells revealed LBR-GFP redistributing into the interconnected ER membrane system in prometaphase, exhibiting the same high mobility and diffusion constant as observed in interphase ER membranes. LBR-GFP rapidly diffused across the cell within the membrane network defined by the ER, suggesting the integrity of the ER was maintained in mitosis, with little or no fragmentation and vesiculation. At the end of mitosis, nuclear membrane reformation coincided with immobilization of LBR-GFP in ER elements at contact sites with chromatin. LBR-GFP-containing ER membranes then wrapped around chromatin over the course of 2-3 min, quickly and efficiently compartmentalizing nuclear material. Expansion of the NE followed over the course of 30-80 min. Thus, selective changes in lateral mobility of LBR-GFP within the ER/NE membrane system form the basis for its localization to the inner nuclear membrane during interphase. Such changes, rather than vesiculation mechanisms, also underlie the redistribution of this molecule during NE disassembly and reformation in mitosis.
Figures




References
-
- Akey CW. Structural plasticity of the nuclear pore complex. J Mol Biol. 1995;248:273–293. - PubMed
-
- Bione S, Maestrini E, Rivella S, Mancini M, Regis S, Romeao G, Toniolo D. Identification of a novel X-linked gene responsible for Emery-Dreifuss muscular dystrophy. Nature Genet. 1994;8:323–329. - PubMed
-
- Bourgeois CA, Hemon D, Bouteille M. Structural relationship between the nucleolus and the nuclear envelope. J Ultrastruct Res. 1979;68:328–340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

