Localization of p21-activated kinase 1 (PAK1) to pinocytic vesicles and cortical actin structures in stimulated cells
- PMID: 9298982
- PMCID: PMC2132543
- DOI: 10.1083/jcb.138.6.1265
Localization of p21-activated kinase 1 (PAK1) to pinocytic vesicles and cortical actin structures in stimulated cells
Abstract
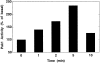
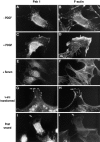
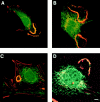
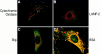
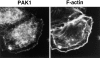
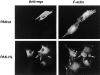
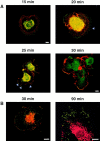
The mechanisms through which the small GTPases Rac1 and Cdc42 regulate the formation of membrane ruffles, lamellipodia, and filopodia are currently unknown. The p21-activated kinases (PAKs) are direct targets of active Rac and Cdc42 which can induce the assembly of polarized cytoskeletal structures when expressed in fibroblasts, suggesting that they may play a role in mediating the effects of these GTPases on cytoskeletal dynamics. We have examined the subcellular localization of endogenous PAK1 in fibroblast cell lines using specific PAK1 antibodies. PAK1 is detected in submembranous vesicles in both unstimulated and stimulated fibroblasts that colocalize with a marker for fluid-phase uptake. In cells stimulated with PDGF, in v-Src-transformed fibroblasts, and in wounded cells, PAK1 redistributed into dorsal and membrane ruffles and into the edges of lamellipodia, where it colocalizes with polymerized actin. PAK1 was also colocalized with F-actin in membrane ruffles extended as a response to constitutive activation of Rac1. PAK1 appears to precede F-actin in translocating to cytoskeletal structures formed at the cell periphery. The association of PAK1 with the actin cytoskeleton is prevented by the actin filament-disrupting agent cytochalasin D and by the phosphatidylinositol 3-kinase inhibitor wortmannin. Co-immunoprecipitation experiments demonstrate an in vivo interaction of PAK1 with filamentous (F)-actin in stimulated cells. Microinjection of a constitutively active PAK1 mutant into Rat-1 fibroblasts overexpressing the insulin receptor (HIRcB cells) induced the formation of F-actin- and PAK1-containing structures reminiscent of dorsal ruffles. These data indicate a close correlation between the subcellular distribution of endogenous PAK1 and the formation of Rac/Cdc42-dependent cytoskeletal structures and support an active role for PAK1 in regulating cortical actin rearrangements.
Figures
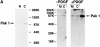
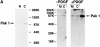


References
-
- Bagrodia S, Derijard B, Davis RJ, Cerione RA. Cdc42 and PAKmediated signaling leads to JUN kinase and p38MAP kinase activation. J Biol Chem. 1995a;270:27995–27998. - PubMed
-
- Bagrodia S, Taylor SJ, Creasy CL, Chernoff J, Cerione RA. Identification of a mouse p21Cdc42/Rac-activated kinase. J Biol Chem. 1995b;270:22731–22737. - PubMed
-
- Bokoch GM, Wang Y, Bohl BP, Sells MA, Quilliam LA, Knaus UG. Interaction of the Nck adapter protein with p21-activated kinase (PAK1) J Biol Chem. 1996;271:25746–25749. - PubMed
-
- Bretscher MS. Getting membrane flow and the cytoskeleton to cooperate in moving cells. Cell. 1996;87:601–606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

